Abstract
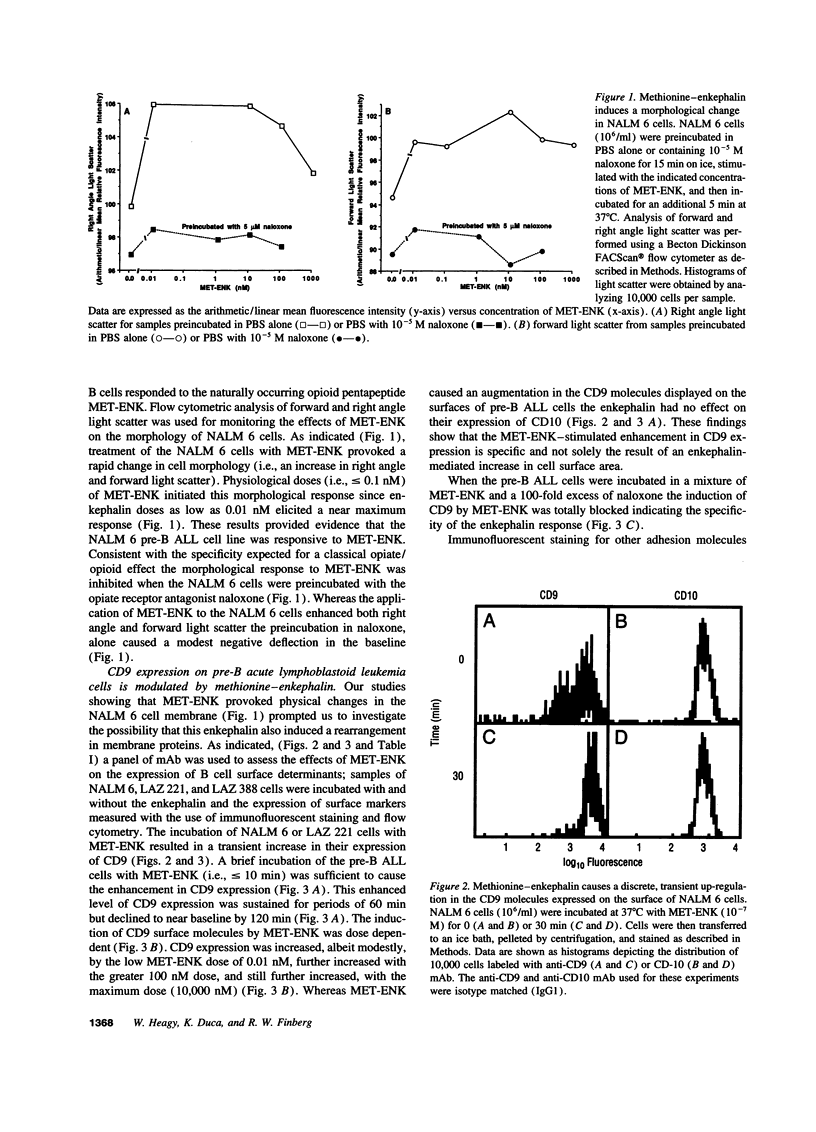
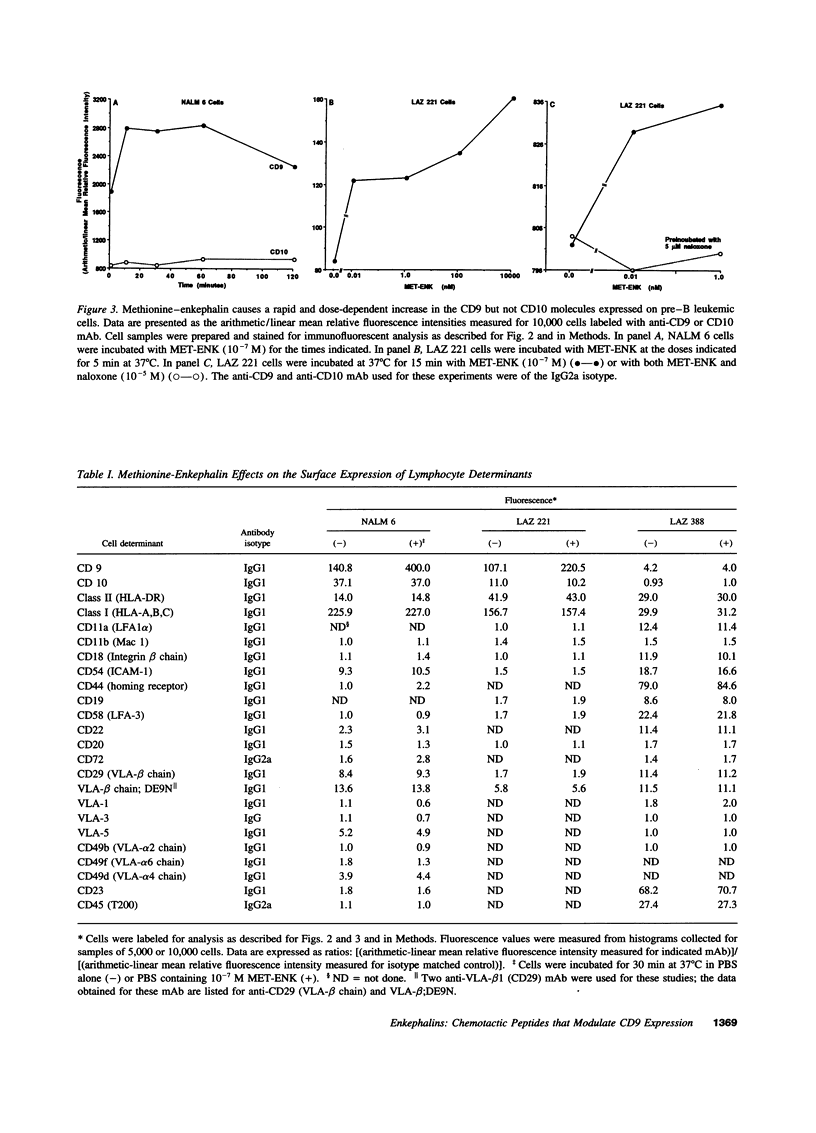
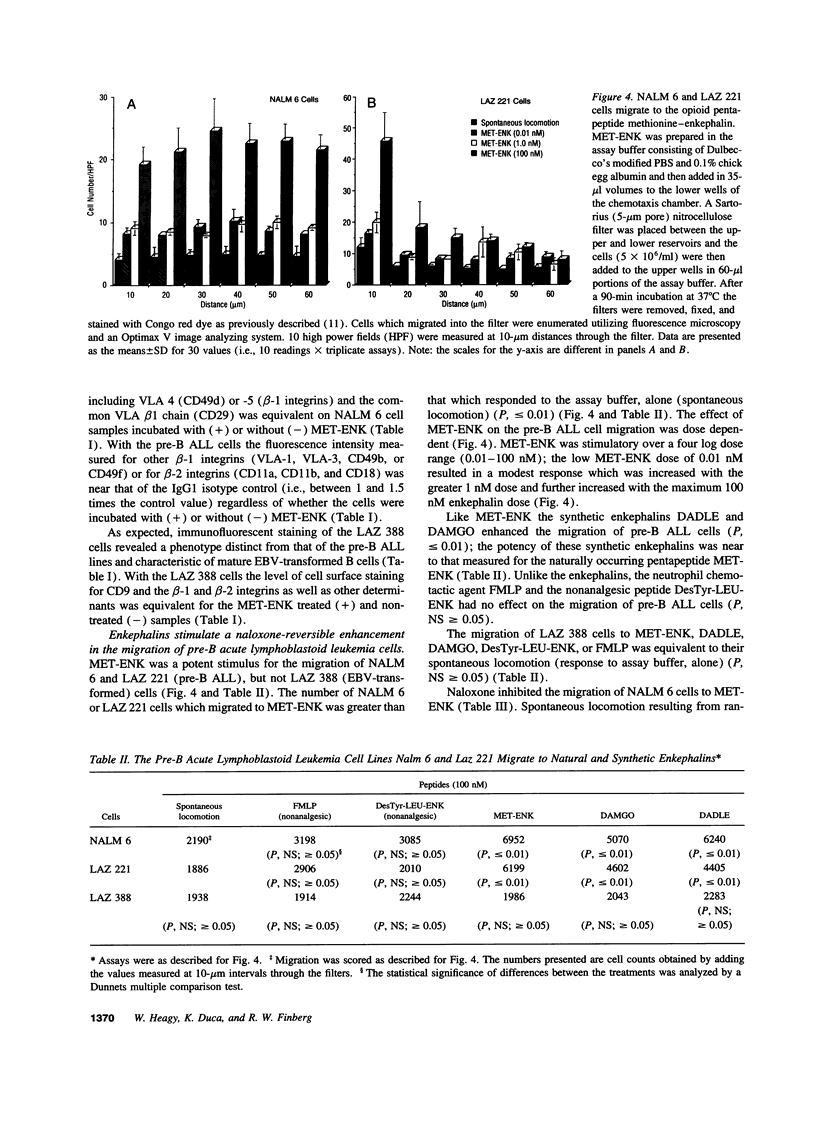
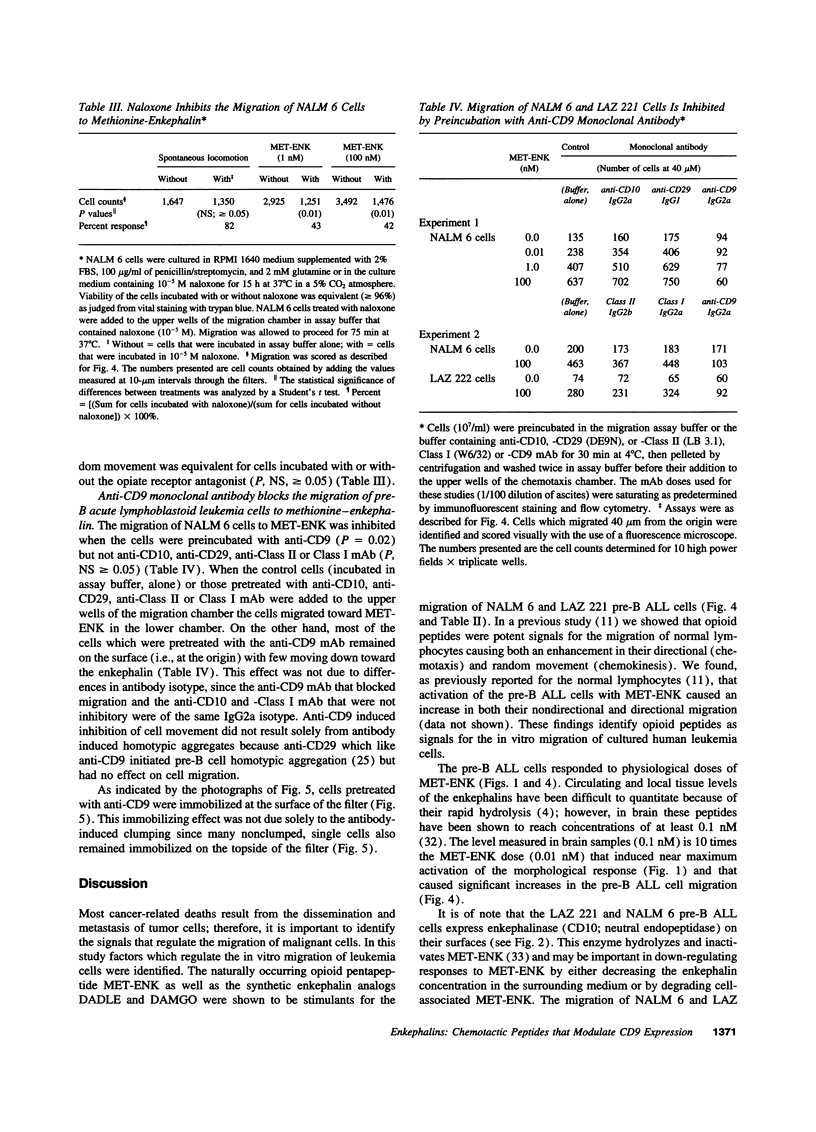
Opioid peptides have been implicated in the regulation of tumor growth and biology; however, little attention has been given to the mechanisms that are involved. In this study we show that physiological concentrations of the endogenous opioid neuropeptide methionine-enkephalin (MET-ENK) and the synthetic enkephalins D-Ala2, Me-Phe4, Gly(ol)5 and D-Ala2, D-Leu5 are stimulants for the in vitro migration of pre-B acute lymphoblastoid leukemia (ALL) cells. Activation of the human pre-B ALL cell lines NALM 6 and LAZ 221 with MET-ENK resulted in both an increase in their migration and an augmentation in the surface expression of the leukemia cell marker CD9. The opiate receptor antagonist naloxone reversed these enkephalin-induced effects on the leukemia cells. When the pre-B ALL cells were preincubated with an anti-CD9 mAb before challenge with MET-ENK their migration to the enkephalin was markedly reduced. These studies show that endogenous and synthetic opioid peptides are stimulants for pre-B ALL cell migration and suggest that CD9 is important in the regulation of leukemia cell motility.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergelson J. M., Shepley M. P., Chan B. M., Hemler M. E., Finberg R. W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992 Mar 27;255(5052):1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- Borboni P., Di Cola G., Sesti G., Marini M. A., Del Porto P., Gilardini Montani M. S., Lauro R., De Pirro R. Beta-endorphin receptors on cultured and freshly isolated lymphocytes from normal subjects. Biochem Biophys Res Commun. 1989 Aug 30;163(1):642–648. doi: 10.1016/0006-291x(89)92185-2. [DOI] [PubMed] [Google Scholar]
- Bradbury L. E., Goldmacher V. S., Tedder T. F. The CD19 signal transduction complex of B lymphocytes. Deletion of the CD19 cytoplasmic domain alters signal transduction but not complex formation with TAPA-1 and Leu 13. J Immunol. 1993 Sep 15;151(6):2915–2927. [PubMed] [Google Scholar]
- Chao C. C., Sharp B. M., Pomeroy C., Filice G. A., Peterson P. K. Lethality of morphine in mice infected with Toxoplasma gondii. J Pharmacol Exp Ther. 1990 Feb;252(2):605–609. [PubMed] [Google Scholar]
- Ebener U., Wehner S., Cinatl J., Gussetis E. S., Kornhuber B. Expression of markers shared between human haematopoietic cells and neuroblastoma cells. Anticancer Res. 1990 Jul-Aug;10(4):887–890. [PubMed] [Google Scholar]
- Falke N. E., Fischer E. G. Cell shape of polymorphonuclear leukocytes is influenced by opioids. Immunobiology. 1985 Jul;169(5):532–539. doi: 10.1016/S0171-2985(85)80007-3. [DOI] [PubMed] [Google Scholar]
- Fischer E. G. Opioid peptides modulate immune functions. A review. Immunopharmacol Immunotoxicol. 1988;10(3):265–326. doi: 10.3109/08923978809041423. [DOI] [PubMed] [Google Scholar]
- Fóris G., Medgyesi G. A., Nagy J. T., Varga Z. Concentration-dependent effect of met-enkephalin on human polymorphonuclear leukocytes. Ann N Y Acad Sci. 1987;496:151–157. doi: 10.1111/j.1749-6632.1987.tb35758.x. [DOI] [PubMed] [Google Scholar]
- Gilmore W., Moloney M., Weiner L. P. The role of opioid peptides in immunomodulation. Ann N Y Acad Sci. 1990;597:252–263. doi: 10.1111/j.1749-6632.1990.tb16174.x. [DOI] [PubMed] [Google Scholar]
- Heagy W., Laurance M., Cohen E., Finberg R. Neurohormones regulate T cell function. J Exp Med. 1990 May 1;171(5):1625–1633. doi: 10.1084/jem.171.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagy W., Shipp M. A., Finberg R. W. Opioid receptor agonists and Ca2+ modulation in human B cell lines. J Immunol. 1992 Dec 15;149(12):4074–4081. [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Ikeyama S., Koyama M., Yamaoko M., Sasada R., Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993 May 1;177(5):1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993 Dec 1;151(11):6470–6481. [PubMed] [Google Scholar]
- Komada Y., Ochiai H., Shimizu K., Azuma E., Kamiya H., Sakurai M. Shedding of CD9 antigen into cerebrospinal fluid by acute lymphoblastic leukemia cells. Blood. 1990 Jul 1;76(1):112–116. [PubMed] [Google Scholar]
- Letarte M., Seehafer J. G., Greaves A., Masellis-Smith A., Shaw A. R. Homotypic aggregation of pre-B leukemic cell lines by antibodies to VLA integrins correlates with their expression of CD9. Leukemia. 1993 Jan;7(1):93–103. [PubMed] [Google Scholar]
- Maneckjee R., Minna J. D. Nonconventional opioid binding sites mediate growth inhibitory effects of methadone on human lung cancer cells. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1169–1173. doi: 10.1073/pnas.89.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J., Lookingland K. J., Moore K. E. Kappa-opioid-receptor-mediated regulation of alpha-melanocyte-stimulating hormone secretion and tuberohypophyseal dopaminergic neuronal activity. Neuroendocrinology. 1990 Aug;52(2):200–205. doi: 10.1159/000125582. [DOI] [PubMed] [Google Scholar]
- Masellis-Smith A., Jensen G. S., Seehafer J. G., Slupsky J. R., Shaw A. R. Anti-CD9 monoclonal antibodies induce homotypic adhesion of pre-B cell lines by a novel mechanism. J Immunol. 1990 Mar 1;144(5):1607–1613. [PubMed] [Google Scholar]
- Mitamura T., Iwamoto R., Umata T., Yomo T., Urabe I., Tsuneoka M., Mekada E. The 27-kD diphtheria toxin receptor-associated protein (DRAP27) from vero cells is the monkey homologue of human CD9 antigen: expression of DRAP27 elevates the number of diphtheria toxin receptors on toxin-sensitive cells. J Cell Biol. 1992 Sep;118(6):1389–1399. doi: 10.1083/jcb.118.6.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M., Koyama M., Seno M., Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991 Dec 1;174(6):1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J. L., Broskie E. N., Ranparia D. J., Sharief Y., Coleman W. B., Smith G. J. Cancer cell motility-inhibitory protein in the Dunning adenocarcinoma model. Cancer Res. 1992 Apr 15;52(8):2349–2352. [PubMed] [Google Scholar]
- Moore T. C. Modification of lymphocyte traffic by vasoactive neurotransmitter substances. Immunology. 1984 Jul;52(3):511–518. [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Klein G. Phenotypic and cytogenetic characteristics of human B-lymphoid cell lines and their relevance for the etiology of Burkitt's lymphoma. Adv Cancer Res. 1982;37:319–380. doi: 10.1016/s0065-230x(08)60886-6. [DOI] [PubMed] [Google Scholar]
- Pasternak G. W. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993 Feb;16(1):1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Molitor T. W., Chao C. C. Mechanisms of morphine-induced immunomodulation. Biochem Pharmacol. 1993 Aug 3;46(3):343–348. doi: 10.1016/0006-2952(93)90508-t. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Benoit P., Billard M., Plaisance S., Prenant M., Uzan G., Boucheix C. Organization of the human CD9 gene. Genomics. 1993 Apr;16(1):132–138. doi: 10.1006/geno.1993.1150. [DOI] [PubMed] [Google Scholar]
- Saland L. C., Van Epps D. E., Ortiz E., Samora A. Acute injections of opiate peptides into the rat cerebral ventricle: a macrophage-like cellular response. Brain Res Bull. 1983 Apr;10(4):523–528. doi: 10.1016/0361-9230(83)90150-8. [DOI] [PubMed] [Google Scholar]
- Seehafer J. G., Shaw A. R. Evidence that the signal-initiating membrane protein CD9 is associated with small GTP-binding proteins. Biochem Biophys Res Commun. 1991 Aug 30;179(1):401–406. doi: 10.1016/0006-291x(91)91384-o. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Shipp M. A., Stefano G. B., D'Adamio L., Switzer S. N., Howard F. D., Sinisterra J., Scharrer B., Reinherz E. L. Downregulation of enkephalin-mediated inflammatory responses by CD10/neutral endopeptidase 24.11. Nature. 1990 Sep 27;347(6291):394–396. doi: 10.1038/347394a0. [DOI] [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibinga N. E., Goldstein A. Opioid peptides and opioid receptors in cells of the immune system. Annu Rev Immunol. 1988;6:219–249. doi: 10.1146/annurev.iy.06.040188.001251. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G., Finney D. A. Neutrophil degranulation detected by right angle light scattering: spectroscopic methods suitable for simultaneous analyses of degranulation or shape change, elastase release, and cell aggregation. J Immunol. 1984 Sep;133(3):1483–1487. [PubMed] [Google Scholar]
- Slupsky J. R., Seehafer J. G., Tang S. C., Masellis-Smith A., Shaw A. R. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989 Jul 25;264(21):12289–12293. [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stein C., Hassan A. H., Lehrberger K., Giefing J., Yassouridis A. Local analgesic effect of endogenous opioid peptides. Lancet. 1993 Aug 7;342(8867):321–324. doi: 10.1016/0140-6736(93)91471-w. [DOI] [PubMed] [Google Scholar]
- Vaughn L. K., Wire W. S., Davis P., Shimohigashi Y., Toth G., Knapp R. J., Hruby V. J., Burks T. F., Yamamura H. I. Differentiation between rat brain and mouse vas deferens delta opioid receptors. Eur J Pharmacol. 1990 Feb 20;177(1-2):99–101. doi: 10.1016/0014-2999(90)90556-l. [DOI] [PubMed] [Google Scholar]
- Wardlaw S. L., Wehrenberg W. B., Ferin M., Carmel P. W., Frantz A. G. High levels of beta-endorphin in hypophyseal portal blood. Endocrinology. 1980 May;106(5):1323–1326. doi: 10.1210/endo-106-5-1323. [DOI] [PubMed] [Google Scholar]
- Yatomi Y., Ozaki Y., Satoh K., Kume S. Anti-CD9 monoclonal antibody elicits staurosporine inhibitable phosphatidylinositol 4,5-bisphosphate hydrolysis, phosphatidylinositol 3,4-bisphosphate synthesis, and protein-tyrosine phosphorylation in human platelets. FEBS Lett. 1993 May 17;322(3):285–290. doi: 10.1016/0014-5793(93)81587-p. [DOI] [PubMed] [Google Scholar]
- Ye S., Applegren R. R., Davis J. M., Cheung H. T. Modulation of lymphocyte motility by beta-endorphin and met-enkephalin. Immunopharmacology. 1989 Mar-Apr;17(2):81–89. doi: 10.1016/0162-3109(89)90053-2. [DOI] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Modulation of murine neuroblastoma in nude mice by opioid antagonists. J Natl Cancer Inst. 1987 Jan;78(1):141–147. doi: 10.1093/jnci/78.1.141. [DOI] [PubMed] [Google Scholar]
- Zola H., Furness V., Barclay S., Zowtyj H., Smith M., Melo J. V., Neoh S. H., Bradley J. The p24 leucocyte membrane antigen: modulation associated with lymphocyte activation and differentiation. Immunol Cell Biol. 1989 Feb;67(Pt 1):63–70. doi: 10.1038/icb.1989.8. [DOI] [PubMed] [Google Scholar]






