Abstract
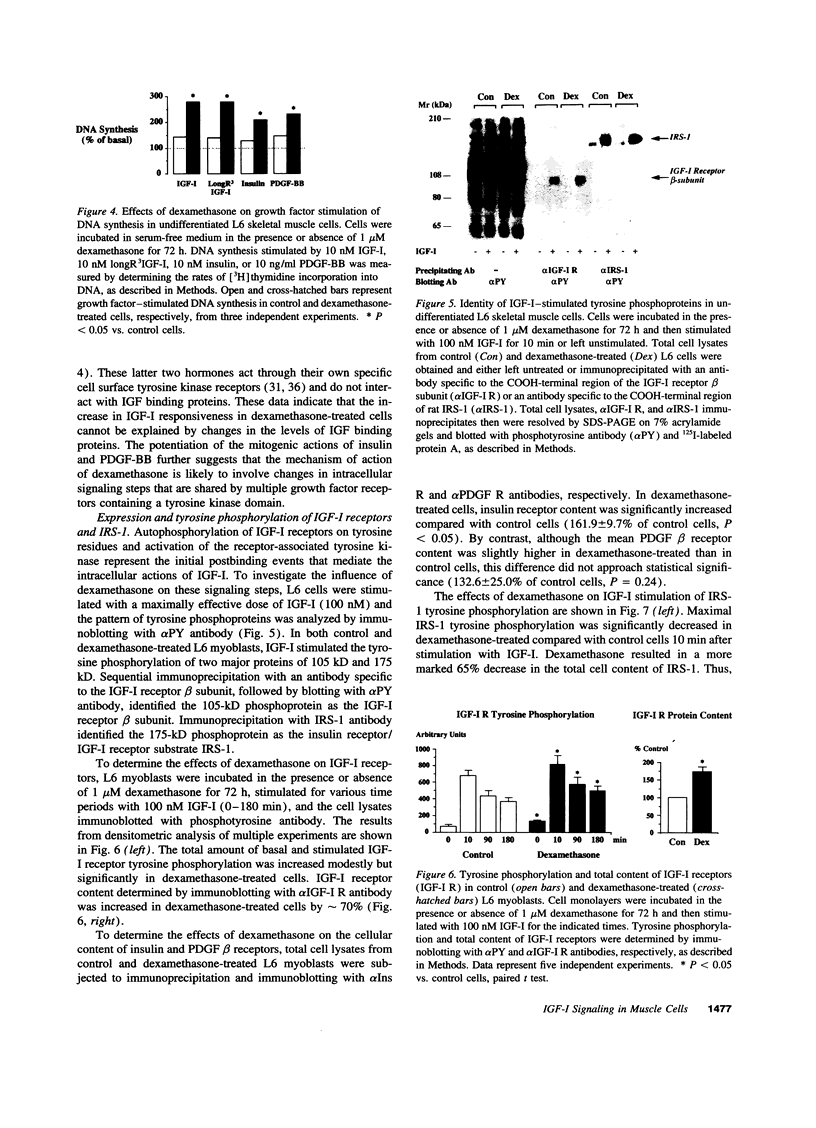
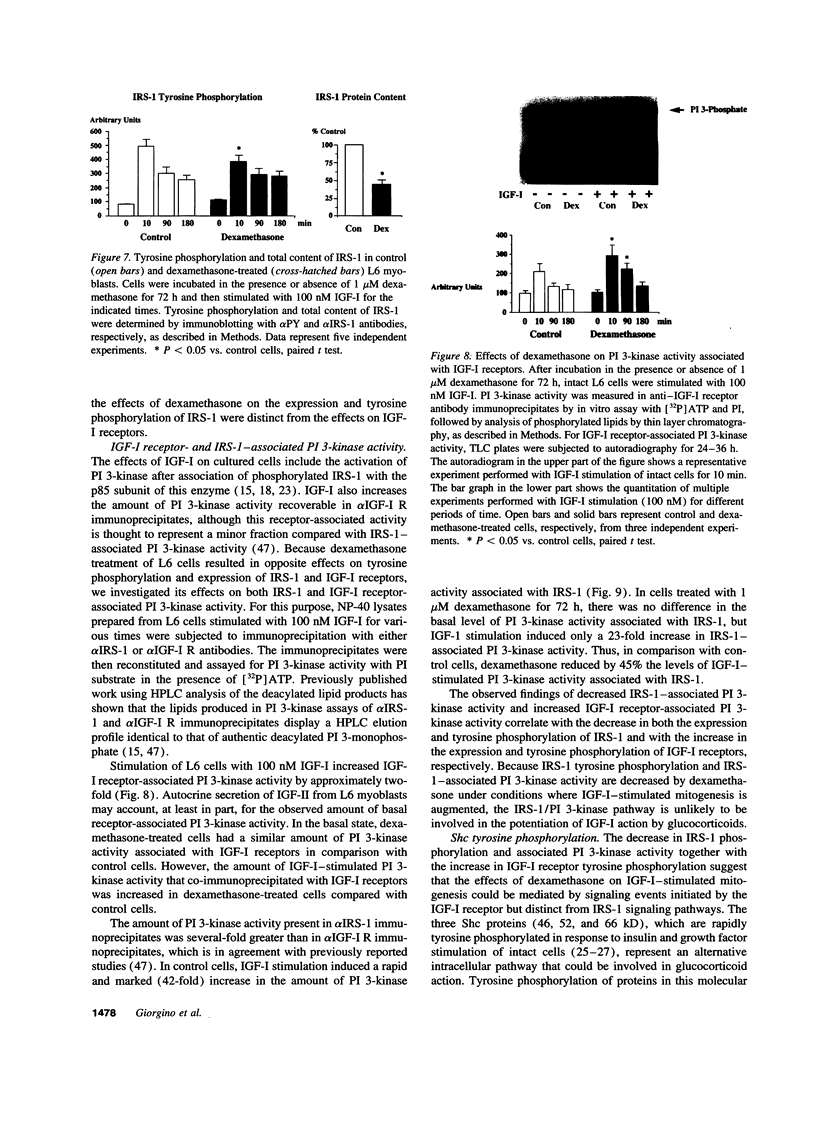
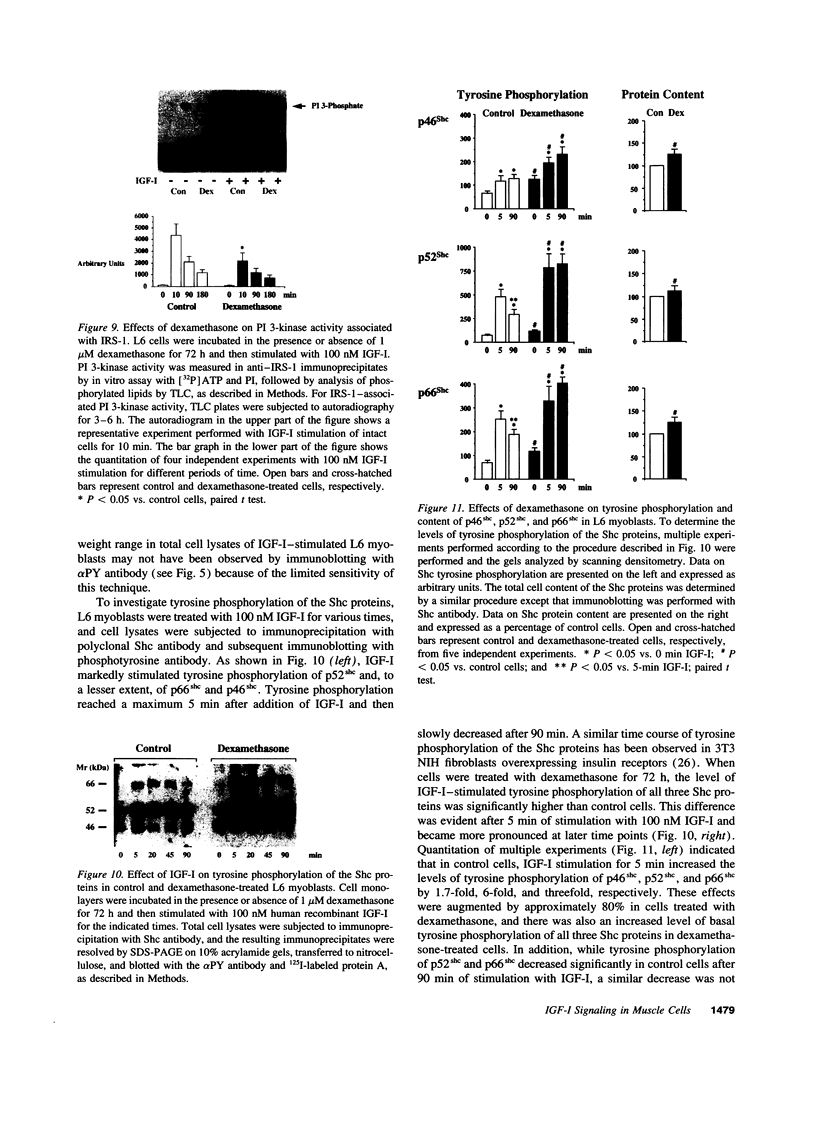
IGF-I stimulation of cell proliferation and c-Fos expression in skeletal muscle cells is markedly enhanced by dexamethasone. The effect of dexamethasone is not mediated by changes in IGF-binding proteins, as evidenced by similar effects of dexamethasone on the actions of insulin, PDGF-BB, and the IGF-I analogue long R3IGF-I. Dexamethasone also does not alter autocrine IGF-II secretion by muscle cells. To investigate the mechanism of the augmentation of IGF-I action, the effects of dexamethasone on intracellular IGF-I signaling pathways were determined. In dexamethasone-treated cells, the levels of IGF-I receptor tyrosine phosphorylation and receptor-associated phosphatidylinositol 3-kinase activity were increased. Dexamethasone-treated cells also showed increased and prolonged tyrosine phosphorylation of the Shc proteins. In contrast, dexamethasone decreased both tyrosine phosphorylation and expression of insulin receptor substrate 1 (IRS-1) and IRS-1-associated phosphatidylinositol 3-kinase activity. Thus, distinct signaling pathways activated by the IGF-I receptor in skeletal muscle cells are differentially regulated by dexamethasone. Potentiation of IGF-I action correlates with increased IGF-I receptor-associated phosphatidylinositol 3-kinase activity and tyrosine phosphorylation of Shc, but appears to be independent of activation of the IRS-1/phosphatidylinositol 3-kinase signaling pathway.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott A. M., Bueno R., Pedrini M. T., Murray J. M., Smith R. J. Insulin-like growth factor I receptor gene structure. J Biol Chem. 1992 May 25;267(15):10759–10763. [PubMed] [Google Scholar]
- Alexandrides T., Moses A. C., Smith R. J. Developmental expression of receptors for insulin, insulin-like growth factor I (IGF-I), and IGF-II in rat skeletal muscle. Endocrinology. 1989 Feb;124(2):1064–1076. doi: 10.1210/endo-124-2-1064. [DOI] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Shoelson S. E., Chin D. J., Sun X. J., Miralpeix M., Hu P., Margolis B., Skolnik E. Y., Schlessinger J. Phosphatidylinositol 3'-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992 Sep;11(9):3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., Myers M. G., Jr, Sun X. J., Chin D. J., Shoelson S. E., Miralpeix M., White M. F. Association of IRS-1 with the insulin receptor and the phosphatidylinositol 3'-kinase. Formation of binary and ternary signaling complexes in intact cells. J Biol Chem. 1993 Apr 15;268(11):8204–8212. [PubMed] [Google Scholar]
- Baker J. B., Barsh G. S., Carney D. H., Cunningham D. D. Dexamethasone modulates binding and action of epidermal growth factor in serum-free cell culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1882–1886. doi: 10.1073/pnas.75.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., Smith R. J. Distinct biologically active receptors for insulin, insulin-like growth factor I, and insulin-like growth factor II in cultured skeletal muscle cells. J Biol Chem. 1985 Dec 15;260(29):15892–15898. [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., Smith R. J. The development of insulin receptors and responsiveness is an early marker of differentiation in the muscle cell line L6. Endocrinology. 1986 Jan;118(1):446–455. doi: 10.1210/endo-118-1-446. [DOI] [PubMed] [Google Scholar]
- Beguinot F., Kahn C. R., Moses A. C., White M. F., Smith R. J. Differentiation-dependent phosphorylation of a 175,000 molecular weight protein in response to insulin and insulin-like growth factor-I in L6 skeletal muscle cells. Endocrinology. 1989 Sep;125(3):1599–1605. doi: 10.1210/endo-125-3-1599. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowsher R. R., Lee W. H., Apathy J. M., O'Brien P. J., Ferguson A. L., Henry D. P. Measurement of insulin-like growth factor-II in physiological fluids and tissues. I. An improved extraction procedure and radioimmunoassay for human and rat fluids. Endocrinology. 1991 Feb;128(2):805–814. doi: 10.1210/endo-128-2-805. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Cascieri M. A., Camacho-Hubner C., McCusker R. H., Bayne M. L. Discrete alterations of the insulin-like growth factor I molecule which alter its affinity for insulin-like growth factor-binding proteins result in changes in bioactivity. J Biol Chem. 1990 Jul 25;265(21):12210–12216. [PubMed] [Google Scholar]
- Condorelli G., Formisano P., Villone G., Smith R. J., Beguinot F. Insulin and insulin-like growth factor I (IGF I) stimulate phosphorylation of a Mr 175,000 cytoskeleton-associated protein in intact FRTL5 cells. J Biol Chem. 1989 Jul 25;264(21):12633–12638. [PubMed] [Google Scholar]
- Conover C. A., Dollar L. A., Hintz R. L., Rosenfeld R. G. Insulin-like growth factor I/somatomedin-C (IGF-I/SM-C) and glucocorticoids synergistically regulate mitosis in competent human fibroblasts. J Cell Physiol. 1983 Aug;116(2):191–197. doi: 10.1002/jcp.1041160210. [DOI] [PubMed] [Google Scholar]
- Conover C. A., Rosenfeld R. G., Hintz R. L. Serum glucocorticoids have persistent and controlling effects on insulinlike growth factor I action under serum-free assay conditions in cultured human fibroblasts. In Vitro Cell Dev Biol. 1989 Jun;25(6):521–527. doi: 10.1007/BF02623564. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Falen S. L., Florini J. R. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology. 1987 Jan;120(1):115–123. doi: 10.1210/endo-120-1-115. [DOI] [PubMed] [Google Scholar]
- Florini J. R. Hormonal control of muscle growth. Muscle Nerve. 1987 Sep;10(7):577–598. doi: 10.1002/mus.880100702. [DOI] [PubMed] [Google Scholar]
- Florini J. R., Magri K. A., Ewton D. Z., James P. L., Grindstaff K., Rotwein P. S. "Spontaneous" differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem. 1991 Aug 25;266(24):15917–15923. [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Giorgetti S., Ballotti R., Kowalski-Chauvel A., Tartare S., Van Obberghen E. The insulin and insulin-like growth factor-I receptor substrate IRS-1 associates with and activates phosphatidylinositol 3-kinase in vitro. J Biol Chem. 1993 Apr 5;268(10):7358–7364. [PubMed] [Google Scholar]
- Giorgetti S., Pelicci P. G., Pelicci G., Van Obberghen E. Involvement of Src-homology/collagen (SHC) proteins in signaling through the insulin receptor and the insulin-like-growth-factor-I-receptor. Eur J Biochem. 1994 Jul 1;223(1):195–202. doi: 10.1111/j.1432-1033.1994.tb18983.x. [DOI] [PubMed] [Google Scholar]
- Giorgino F., Almahfouz A., Goodyear L. J., Smith R. J. Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Invest. 1993 May;91(5):2020–2030. doi: 10.1172/JCI116424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgino F., Chen J. H., Smith R. J. Changes in tyrosine phosphorylation of insulin receptors and a 170,000 molecular weight nonreceptor protein in vivo in skeletal muscle of streptozotocin-induced diabetic rats: effects of insulin and glucose. Endocrinology. 1992 Mar;130(3):1433–1444. doi: 10.1210/endo.130.3.1531627. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Stimulation of division of sparse and confluent 3T3 cell populations by a fibroblast growth factor, dexamethasone, and insulin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4584–4588. doi: 10.1073/pnas.71.11.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Zhang W., Olson E. N. Dexamethasone-dependent inhibition of differentiation of C2 myoblasts bearing steroid-inducible N-ras oncogenes. J Cell Biol. 1988 Jun;106(6):2127–2137. doi: 10.1083/jcb.106.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Jin P., Sejersen T., Ringertz N. R. Recombinant platelet-derived growth factor-BB stimulates growth and inhibits differentiation of rat L6 myoblasts. J Biol Chem. 1991 Jan 15;266(2):1245–1249. [PubMed] [Google Scholar]
- Kaburagi Y., Momomura K., Yamamoto-Honda R., Tobe K., Tamori Y., Sakura H., Akanuma Y., Yazaki Y., Kadowaki T. Site-directed mutagenesis of the juxtamembrane domain of the human insulin receptor. J Biol Chem. 1993 Aug 5;268(22):16610–16622. [PubMed] [Google Scholar]
- Kapeller R., Chakrabarti R., Cantley L., Fay F., Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphatidylinositol-3' kinase complexes: potential interactions with the microtubule cytoskeleton. Mol Cell Biol. 1993 Oct;13(10):6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S. R., Kitagawa K., Aebersold R., Lienhard G. E., Garner C. W. Isolation and characterization of the 160,000-Da phosphotyrosyl protein, a putative participant in insulin signaling. J Biol Chem. 1991 Jul 15;266(20):12817–12820. [PubMed] [Google Scholar]
- Kovacina K. S., Roth R. A. Identification of SHC as a substrate of the insulin receptor kinase distinct from the GAP-associated 62 kDa tyrosine phosphoprotein. Biochem Biophys Res Commun. 1993 May 14;192(3):1303–1311. doi: 10.1006/bbrc.1993.1558. [DOI] [PubMed] [Google Scholar]
- Larson D. E., Xie W., Glibetic M., O'Mahony D., Sells B. H., Rothblum L. I. Coordinated decreases in rRNA gene transcription factors and rRNA synthesis during muscle cell differentiation. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7933–7936. doi: 10.1073/pnas.90.17.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCusker R. H., Camacho-Hübner C., Clemmons D. R. Identification of the types of insulin-like growth factor-binding proteins that are secreted by muscle cells in vitro. J Biol Chem. 1989 May 15;264(14):7795–7800. [PubMed] [Google Scholar]
- McGlade J., Cheng A., Pelicci G., Pelicci P. G., Pawson T. Shc proteins are phosphorylated and regulated by the v-Src and v-Fps protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8869–8873. doi: 10.1073/pnas.89.19.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Jr, Sun X. J., Cheatham B., Jachna B. R., Glasheen E. M., Backer J. M., White M. F. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3'-kinase. Endocrinology. 1993 Apr;132(4):1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- Myers M. G., Jr, Sun X. J., White M. F. The IRS-1 signaling system. Trends Biochem Sci. 1994 Jul;19(7):289–293. doi: 10.1016/0968-0004(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Ohmichi M., Matuoka K., Takenawa T., Saltiel A. R. Growth factors differentially stimulate the phosphorylation of Shc proteins and their association with Grb2 in PC-12 pheochromocytoma cells. J Biol Chem. 1994 Jan 14;269(2):1143–1148. [PubMed] [Google Scholar]
- Orlowski C. C., Ooi G. T., Rechler M. M. Dexamethasone stimulates transcription of the insulin-like growth factor-binding protein-1 gene in H4-II-E rat hepatoma cells. Mol Endocrinol. 1990 Oct;4(10):1592–1599. doi: 10.1210/mend-4-10-1592. [DOI] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Prigent S. A., Gullick W. J. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994 Jun 15;13(12):2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk G. J., McGlade J., Pelicci G., Pawson T., Bos J. L. Insulin-induced phosphorylation of the 46- and 52-kDa Shc proteins. J Biol Chem. 1993 Mar 15;268(8):5748–5753. [PubMed] [Google Scholar]
- Pronk G. J., de Vries-Smits A. M., Buday L., Downward J., Maassen J. A., Medema R. H., Bos J. L. Involvement of Shc in insulin- and epidermal growth factor-induced activation of p21ras. Mol Cell Biol. 1994 Mar;14(3):1575–1581. doi: 10.1128/mcb.14.3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D. W., Saltiel A. R., Majumdar M., Decker S. J., Olefsky J. M. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):797–801. doi: 10.1073/pnas.91.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal S. M., Brunetti A., Brown E. J., Mamula P. W., Goldfine I. D. Regulation of insulin-like growth factor (IGF) I receptor expression during muscle cell differentiation. Potential autocrine role of IGF-II. J Clin Invest. 1991 Apr;87(4):1212–1219. doi: 10.1172/JCI115121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P. L., Lane W. S., Karasik A., Backer J., White M., Kahn C. R. Purification and partial sequence analysis of pp185, the major cellular substrate of the insulin receptor tyrosine kinase. J Biol Chem. 1991 May 5;266(13):8302–8311. [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. Stimulation of tyrosine-specific phosphorylation in vitro by insulin-like growth factor I. 1983 Sep 29-Oct 5Nature. 305(5933):438–440. doi: 10.1038/305438a0. [DOI] [PubMed] [Google Scholar]
- Sasaoka T., Rose D. W., Jhun B. H., Saltiel A. R., Draznin B., Olefsky J. M. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem. 1994 May 6;269(18):13689–13694. [PubMed] [Google Scholar]
- Segatto O., Pelicci G., Giuli S., Digiesi G., Di Fiore P. P., McGlade J., Pawson T., Pelicci P. G. Shc products are substrates of erbB-2 kinase. Oncogene. 1993 Aug;8(8):2105–2112. [PubMed] [Google Scholar]
- Skolnik E. Y., Lee C. H., Batzer A., Vicentini L. M., Zhou M., Daly R., Myers M. J., Jr, Backer J. M., Ullrich A., White M. F. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993 May;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Sun X. J., Crimmins D. L., Myers M. G., Jr, Miralpeix M., White M. F. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993 Dec;13(12):7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. J., Rothenberg P., Kahn C. R., Backer J. M., Araki E., Wilden P. A., Cahill D. A., Goldstein B. J., White M. F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991 Jul 4;352(6330):73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- Tollefsen S. E., Lajara R., McCusker R. H., Clemmons D. R., Rotwein P. Insulin-like growth factors (IGF) in muscle development. Expression of IGF-I, the IGF-I receptor, and an IGF binding protein during myoblast differentiation. J Biol Chem. 1989 Aug 15;264(23):13810–13817. [PubMed] [Google Scholar]
- Turnbow M. A., Keller S. R., Rice K. M., Garner C. W. Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J Biol Chem. 1994 Jan 28;269(4):2516–2520. [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R. J., Bodenburg Y. H., Nagamani M., Peirce J. Dexamethasone potentiates IGF-I actions in porcine granulosa cells. Am J Physiol. 1994 Jul;267(1 Pt 1):E115–E123. doi: 10.1152/ajpendo.1994.267.1.E115. [DOI] [PubMed] [Google Scholar]
- Van Horn D. J., Myers M. G., Jr, Backer J. M. Direct activation of the phosphatidylinositol 3'-kinase by the insulin receptor. J Biol Chem. 1994 Jan 7;269(1):29–32. [PubMed] [Google Scholar]
- Wang L. M., Myers M. G., Jr, Sun X. J., Aaronson S. A., White M., Pierce J. H. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science. 1993 Sep 17;261(5128):1591–1594. doi: 10.1126/science.8372354. [DOI] [PubMed] [Google Scholar]
- Waters S. B., Yamauchi K., Pessin J. E. Functional expression of insulin receptor substrate-1 is required for insulin-stimulated mitogenic signaling. J Biol Chem. 1993 Oct 25;268(30):22231–22234. [PubMed] [Google Scholar]
- Wu R., Wolfe R. A., Sato G. H. Distinctive effects of hydrocortisone on the modulation of EGF binding and cell growth in HeLa cells grown in defined medium. J Cell Physiol. 1981 Jul;108(1):83–90. doi: 10.1002/jcp.1041080111. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Lapetina E. G., Moxham C. P. Insulin like growth factor-I induces limited association of phosphatidylinositol 3-kinase to its receptor. Endocrinology. 1992 Mar;130(3):1490–1498. doi: 10.1210/endo.130.3.1311242. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]