Abstract
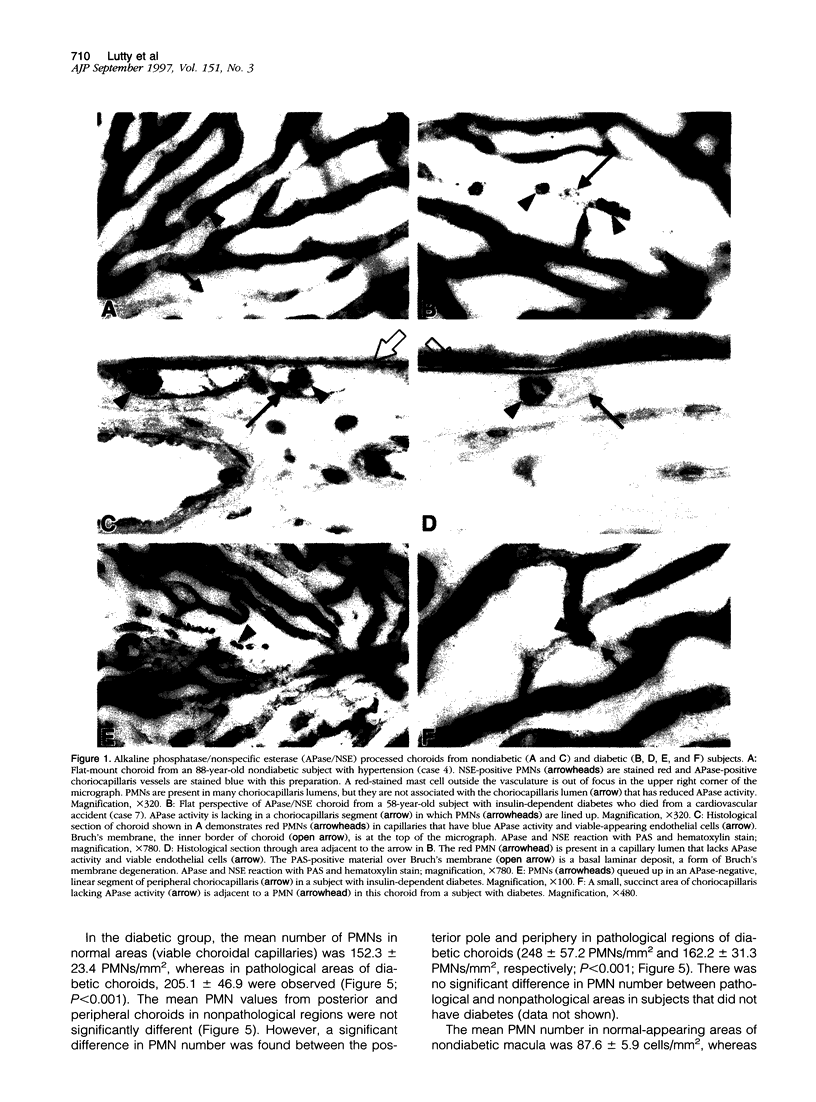
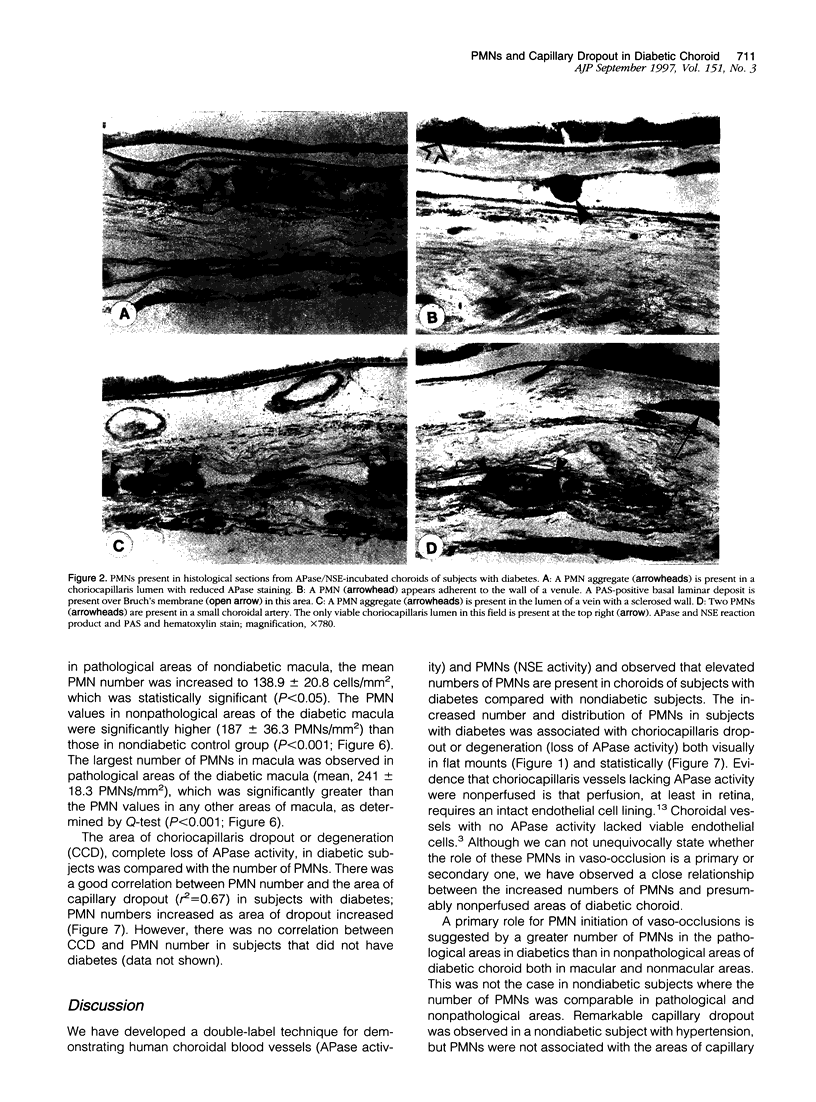
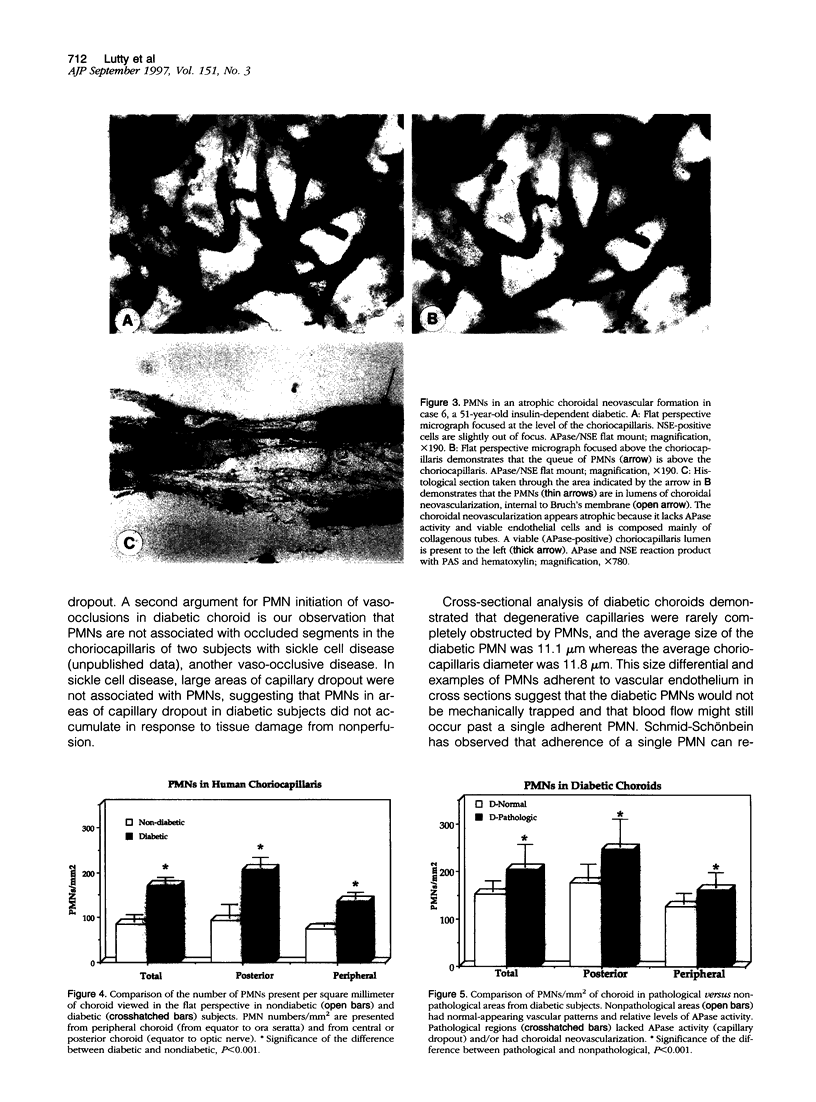
Capillary dropout is an initial event in diabetic retinopathy, but the etiology is unknown. Recent evidence suggests that similar events may occur in the diabetic choroid. We have developed a method to evaluate the relationship between the polymorphonuclear leukocytes (PMNs) and capillary dropout in the human diabetic choroid using alkaline phosphatase (APase) histochemistry to label blood vessels and nonspecific esterase activity to identify PMNs. The number and distribution of PMNs in diabetic and nondiabetic choroidal capillaries (choriocapillaris) were analyzed in the flat perspective and the tissue then flat embedded in glycol methacrylate for histological sectioning. The total number of PMNs was increased within the choriocapillaris in five diabetic eyes (170.9 +/- 12.9 PMNs/mm2 of choroid) compared with five nondiabetic eyes (84.2 +/- 16.9 PMNs/mm2; P < 0.001). PMNs were almost always within blood vessel lumens and not in interstitial tissue. In the diabetic choroid, increased numbers of PMNs were present in areas of choriocapillaris with pathological changes (loss in APase activity and choroidal neovascularization) compared with nonpathological choriocapillaris (205.1 +/- 46.9 PMNs/mm2 in pathological versus 152.3 +/- 23.4 PMNs/mm2 in nonpathological areas; P < 0.001). PMNs were often queued up within the lumens of capillaries, demonstrating loss in APase activity. We have observed an increased number of PMNs in diabetic choroid compared with control nondiabetic choroids, and PMNs in diabetic choroid were associated with loss in APase activity, which was related to loss in viable endothelial cells. The results suggest that PMNs contribute to vaso-occlusive processes and endothelial cell injury in the diabetic choroid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresnick G. H., De Venecia G., Myers F. L., Harris J. A., Davis M. D. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975 Dec;93(12):1300–1310. doi: 10.1001/archopht.1975.01010020934002. [DOI] [PubMed] [Google Scholar]
- Cavallo M. G., Pozzilli P., Bird C., Wadhwa M., Meager A., Visalli N., Gearing A. J., Andreani D., Thorpe R. Cytokines in sera from insulin-dependent diabetic patients at diagnosis. Clin Exp Immunol. 1991 Nov;86(2):256–259. doi: 10.1111/j.1365-2249.1991.tb05806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm L. J., Schultze A. E., Roth R. A. Activated neutrophils injure the isolated, perfused rat liver by an oxygen radical-dependent mechanism. Am J Pathol. 1991 Nov;139(5):1009–1020. [PMC free article] [PubMed] [Google Scholar]
- Elliott M. J., Finn A. H. Interaction between neutrophils and endothelium. Ann Thorac Surg. 1993 Dec;56(6):1503–1508. doi: 10.1016/0003-4975(93)90741-y. [DOI] [PubMed] [Google Scholar]
- Freedman S. F., Hatchell D. L. Enhanced superoxide radical production by stimulated polymorphonuclear leukocytes in a cat model of diabetes. Exp Eye Res. 1992 Nov;55(5):767–773. doi: 10.1016/0014-4835(92)90181-q. [DOI] [PubMed] [Google Scholar]
- Inés Barañao R., Garberi J. C., Tesone P. A., Rumi L. S. Evaluation of neutrophil activity and circulating immune complexes levels in diabetic patients. Horm Metab Res. 1987 Aug;19(8):371–374. doi: 10.1055/s-2007-1011827. [DOI] [PubMed] [Google Scholar]
- Kantar A., Giorgi P. L., Curatola G., Fiorini R. Alterations in membrane fluidity of diabetic polymorphonuclear leukocytes. Biochem Med Metab Biol. 1991 Dec;46(3):422–426. doi: 10.1016/0885-4505(91)90090-8. [DOI] [PubMed] [Google Scholar]
- Kelly L. W., Barden C. A., Tiedeman J. S., Hatchell D. L. Alterations in viscosity and filterability of whole blood and blood cell subpopulations in diabetic cats. Exp Eye Res. 1993 Mar;56(3):341–347. doi: 10.1006/exer.1993.1044. [DOI] [PubMed] [Google Scholar]
- Kim J. A., Berliner J. A., Natarajan R. D., Nadler J. L. Evidence that glucose increases monocyte binding to human aortic endothelial cells. Diabetes. 1994 Sep;43(9):1103–1107. doi: 10.2337/diab.43.9.1103. [DOI] [PubMed] [Google Scholar]
- Kohner E. M., Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970 Mar;69(3):403–414. doi: 10.1016/0002-9394(70)92273-7. [DOI] [PubMed] [Google Scholar]
- Kohner E. M., Porta M. Vascular abnormalities in diabetes and their treatment. Trans Ophthalmol Soc U K. 1980 Sep;100(3):440–444. [PubMed] [Google Scholar]
- Lampeter E. R., Kishimoto T. K., Rothlein R., Mainolfi E. A., Bertrams J., Kolb H., Martin S. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992 Dec;41(12):1668–1671. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- Langham M. E., Grebe R., Hopkins S., Marcus S., Sebag M. Choroidal blood flow in diabetic retinopathy. Exp Eye Res. 1991 Feb;52(2):167–173. doi: 10.1016/0014-4835(91)90256-e. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Lovasik J. V., Kergoat H. Electroretinographic results and ocular vascular perfusion in type 1 diabetes. Invest Ophthalmol Vis Sci. 1993 Apr;34(5):1731–1743. [PubMed] [Google Scholar]
- Lutty G. A., McLeod D. S. A new technique for visualization of the human retinal vasculature. Arch Ophthalmol. 1992 Feb;110(2):267–276. doi: 10.1001/archopht.1992.01080140123039. [DOI] [PubMed] [Google Scholar]
- McLeod D. S., Lefer D. J., Merges C., Lutty G. A. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995 Sep;147(3):642–653. [PMC free article] [PubMed] [Google Scholar]
- McLeod D. S., Lutty G. A. High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci. 1994 Oct;35(11):3799–3811. [PubMed] [Google Scholar]
- Schröder S., Palinski W., Schmid-Schönbein G. W. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991 Jul;139(1):81–100. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Vermes I., Steinmetz E. T., Zeyen L. J., van der Veen E. A. Rheological properties of white blood cells are changed in diabetic patients with microvascular complications. Diabetologia. 1987 Jun;30(6):434–436. doi: 10.1007/BF00292548. [DOI] [PubMed] [Google Scholar]
- Wierusz-Wysocka B., Wysocki H., Siekierka H., Wykretowicz A., Szczepanik A., Klimas R. Evidence of polymorphonuclear neutrophils (PMN) activation in patients with insulin-dependent diabetes mellitus. J Leukoc Biol. 1987 Nov;42(5):519–523. doi: 10.1002/jlb.42.5.519. [DOI] [PubMed] [Google Scholar]
- Yanoff M. Ocular pathology of diabetes mellitus. Am J Ophthalmol. 1969 Jan;67(1):21–38. doi: 10.1016/0002-9394(69)90004-x. [DOI] [PubMed] [Google Scholar]