Abstract
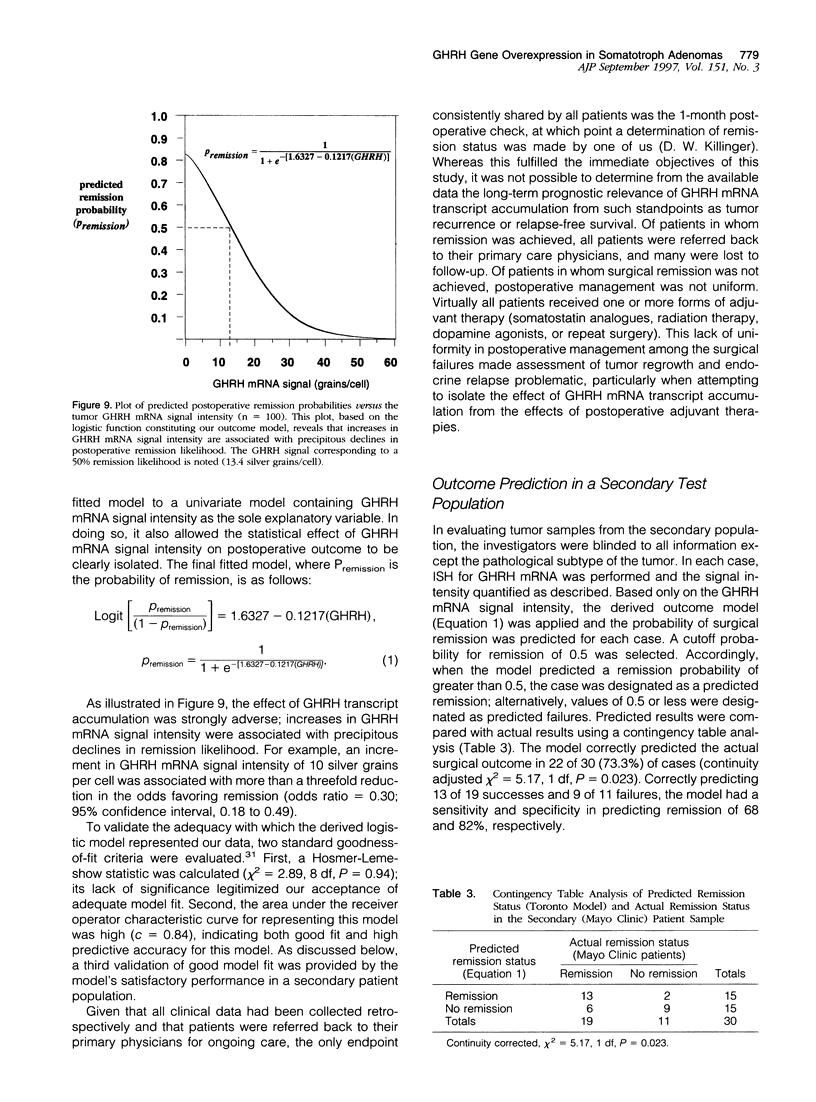
The clinical behavior of growth hormone (GH)-producing pituitary tumors is known to vary greatly; however, the events underlying this variability remain poorly understood. Herein we demonstrate that tumor overexpression of the GH-releasing hormone (GHRH) gene is one prognostically informative event associated with the clinical aggressiveness of somatotroph pituitary tumors. Accumulation of GHRH mRNA transcripts was demonstrated in 91 of a consecutive series of 100 somatotroph tumors by in situ hybridization; these findings were corroborated by Northern analysis and reverse transcriptase polymerase chain reaction, and protein translation was confirmed by Western blotting. By comparison, transcript accumulation was absent or negligibly low in 30 normal pituitary glands. GHRH transcripts were found to preferentially accumulate among clinically aggressive tumors. Specifically, GHRH mRNA signal intensity was 1) linearly correlated with Ki-67 tumor growth fractions (r = 0.71; P < 0.001), 2) linearly correlated with preoperative serum GH levels (r = 0.56; p = 0.01), 3) higher among invasive tumors (P < 0.001), and 4) highest in those tumors in which post-operative remission was not achieved (P < 0.001). Using multivariate logistic regression, a model of postoperative remission likelihood was derived wherein remission was defined by the single criterion of suppressibility of GH levels to less than 2 ng/ml during an oral glucose tolerance test. In this outcome model, GHRH mRNA signal intensity proved to be the most important explanatory variable overall, eclipsing any and all conventional clinicopathological predictors as the single most significant predictor of postoperative remission; increases in GHRH mRNA signal were associated with marked declines in remission likelihood. The generalizability of this outcome model was further validated by the model's significant performance in predicting postoperative remission in a random sample of 30 somatotroph tumors treated at another institution. These data indicate that overexpression of GHRH gene is an event associated with the neoplastic progression and clinical aggressiveness of somatotroph adenomas. More generally, these data merge essential elements of the hypothalamic and pituitary hypotheses of pituitary tumorigenesis, providing for a more unified concept of neoplastic progression in the pituitary.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asa S. L., Kovacs K., Stefaneanu L., Horvath E., Billestrup N., Gonzalez-Manchon C., Vale W. Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology. 1992 Nov;131(5):2083–2089. doi: 10.1210/endo.131.5.1425411. [DOI] [PubMed] [Google Scholar]
- Benlot C., Pagesy P., Peillon F., Joubert-Bression D. Growth hormone-releasing hormone and somatostatin in normal and tumoral human pituitaries. Peptides. 1991 Sep-Oct;12(5):945–950. doi: 10.1016/0196-9781(91)90042-n. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Mitchell R. L., Vale W., Verma I. M. Growth hormone-releasing factor induces c-fos expression in cultured primary pituitary cells. Mol Endocrinol. 1987 Apr;1(4):300–305. doi: 10.1210/mend-1-4-300. [DOI] [PubMed] [Google Scholar]
- Billestrup N., Swanson L. W., Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6854–6857. doi: 10.1073/pnas.83.18.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Faglia G., Arosio M., Bazzoni N. Ectopic acromegaly. Endocrinol Metab Clin North Am. 1992 Sep;21(3):575–595. [PubMed] [Google Scholar]
- Faglia G., Spada A. The role of the hypothalamus in pituitary neoplasia. Baillieres Clin Endocrinol Metab. 1995 Apr;9(2):225–242. doi: 10.1016/s0950-351x(95)80306-8. [DOI] [PubMed] [Google Scholar]
- Fahlbusch R., Honegger J., Buchfelder M. Surgical management of acromegaly. Endocrinol Metab Clin North Am. 1992 Sep;21(3):669–692. [PubMed] [Google Scholar]
- Frohman L. A., Downs T. R., Chomczynski P. Regulation of growth hormone secretion. Front Neuroendocrinol. 1992 Oct;13(4):344–405. [PubMed] [Google Scholar]
- Frohman L. A., Jansson J. O. Growth hormone-releasing hormone. Endocr Rev. 1986 Aug;7(3):223–253. doi: 10.1210/edrv-7-3-223. [DOI] [PubMed] [Google Scholar]
- Gaylinn B. D., Harrison J. K., Zysk J. R., Lyons C. E., Lynch K. R., Thorner M. O. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone. Mol Endocrinol. 1993 Jan;7(1):77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- Hardy J. Transphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg. 1969;16:185–217. doi: 10.1093/neurosurgery/16.cn_suppl_1.185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981 Jun;75(6):816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Joubert D., Benlot C., Lagoguey A., Garnier P., Brandi A. M., Gautron J. P., Legrand J. C., Peillon F. Normal and growth hormone (GH)-secreting adenomatous human pituitaries release somatostatin and GH-releasing hormone. J Clin Endocrinol Metab. 1989 Mar;68(3):572–577. doi: 10.1210/jcem-68-3-572. [DOI] [PubMed] [Google Scholar]
- Kawamoto K., Uozumi T., Arita K., Takechi A., Kawamoto H. Secretion of growth hormone (GH)-releasing hormone by GH-producing pituitary adenoma assessed by cell immunoblot analysis. Endocr J. 1995 Feb;42(1):89–93. doi: 10.1507/endocrj.42.89. [DOI] [PubMed] [Google Scholar]
- Klibanski A., Zervas N. T. Diagnosis and management of hormone-secreting pituitary adenomas. N Engl J Med. 1991 Mar 21;324(12):822–831. doi: 10.1056/NEJM199103213241207. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Harsh G., Lyons J., Davis R. L., McCormick F., Bourne H. R. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990 Dec;71(6):1416–1420. doi: 10.1210/jcem-71-6-1416. [DOI] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Lei T., Adams E. F., Buchfelder M., Fahlbusch R. Relationship between protein kinase C and adenylyl cyclase activity in the regulation of growth hormone secretion by human pituitary somatotrophinomas. Neurosurgery. 1996 Sep;39(3):569–576. doi: 10.1097/00006123-199609000-00027. [DOI] [PubMed] [Google Scholar]
- Levy A., Lightman S. L. Growth hormone-releasing hormone transcripts in human pituitary adenomas. J Clin Endocrinol Metab. 1992 Jun;74(6):1474–1476. doi: 10.1210/jcem.74.6.1350590. [DOI] [PubMed] [Google Scholar]
- Levy A., Lightman S. L. The pathogenesis of pituitary adenomas. Clin Endocrinol (Oxf) 1993 Jun;38(6):559–570. doi: 10.1111/j.1365-2265.1993.tb02136.x. [DOI] [PubMed] [Google Scholar]
- Levy L., Bourdais J., Mouhieddine B., Benlot C., Villares S., Cohen P., Peillon F., Joubert D. Presence and characterization of the somatostatin precursor in normal human pituitaries and in growth hormone secreting adenomas. J Clin Endocrinol Metab. 1993 Jan;76(1):85–90. doi: 10.1210/jcem.76.1.8093621. [DOI] [PubMed] [Google Scholar]
- Li J. Y., Pagesy P., Berthet M., Racadot O., Kujas M., Racadot J., Peillon F. Somatostatin cells in human somatotropic adenomas. Virchows Arch A Pathol Anat Histopathol. 1992;420(1):95–101. doi: 10.1007/BF01605990. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Chang A., Kulig E., Camper S. A., Ross B. D., Downs T. R., Frohman L. A. Morphologic effects of hGRH gene expression on the pituitary, liver, and pancreas of MT-hGRH transgenic mice. An in situ hybridization analysis. Am J Pathol. 1992 Oct;141(4):895–906. [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Godfrey P. A., Suhr S. T., Kulik D. J., Rahal J. O. Growth hormone-releasing hormone: synthesis and signaling. Recent Prog Horm Res. 1995;50:35–73. doi: 10.1016/b978-0-12-571150-0.50007-x. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Vale W., Rivier J., Rosenfeld M. G., Evans R. M. Expression-cloning and sequence of a cDNA encoding human growth hormone-releasing factor. Nature. 1983 Nov 3;306(5938):86–88. doi: 10.1038/306086a0. [DOI] [PubMed] [Google Scholar]
- Melmed S. Acromegaly. N Engl J Med. 1990 Apr 5;322(14):966–977. doi: 10.1056/NEJM199004053221405. [DOI] [PubMed] [Google Scholar]
- Melmed S. Etiology of pituitary acromegaly. Endocrinol Metab Clin North Am. 1992 Sep;21(3):539–551. [PubMed] [Google Scholar]
- Miller G. M., Alexander J. M., Klibanski A. Gonadotropin-releasing hormone messenger RNA expression in gonadotroph tumors and normal human pituitary. J Clin Endocrinol Metab. 1996 Jan;81(1):80–83. doi: 10.1210/jcem.81.1.8550798. [DOI] [PubMed] [Google Scholar]
- Molitch M. E. Clinical manifestations of acromegaly. Endocrinol Metab Clin North Am. 1992 Sep;21(3):597–614. [PubMed] [Google Scholar]
- Mouhieddine O. E., Levy L., Benlot C., Peillon F., Joubert D. Growth hormone (GH)-releasing hormone tonically inhibits in vitro endogenous somatostatin in human GH-secreting tumors. J Clin Endocrinol Metab. 1995 May;80(5):1691–1695. doi: 10.1210/jcem.80.5.7745020. [DOI] [PubMed] [Google Scholar]
- Nabarro J. D. Acromegaly. Clin Endocrinol (Oxf) 1987 Apr;26(4):481–512. doi: 10.1111/j.1365-2265.1987.tb00805.x. [DOI] [PubMed] [Google Scholar]
- Pagesy P., Croissandeau G., Le Dafniet M., Peillon F., Li J. Y. Detection of thyrotropin-releasing hormone (TRH) mRNA by the reverse transcription-polymerase chain reaction in the human normal and tumoral anterior pituitary. Biochem Biophys Res Commun. 1992 Jan 15;182(1):182–187. doi: 10.1016/s0006-291x(05)80128-7. [DOI] [PubMed] [Google Scholar]
- Pagesy P., Li J. Y., Rentier-Delrue F., Delalande O., Le Bouc Y., Kujas M., Joubert D., Martial J., Peillon F. Growth hormone and somatostatin gene expression in pituitary adenomas with active acromegaly and minimal plasma growth hormone elevation. Acta Endocrinol (Copenh) 1990 Jun;122(6):745–752. doi: 10.1530/acta.0.1220745. [DOI] [PubMed] [Google Scholar]
- Rauch C., Li J. Y., Croissandeau G., Berthet M., Peillon F., Pagesy P. Characterization and localization of an immunoreactive growth hormone-releasing hormone precursor form in normal and tumoral human anterior pituitaries. Endocrinology. 1995 Jun;136(6):2594–2601. doi: 10.1210/endo.136.6.7750482. [DOI] [PubMed] [Google Scholar]
- Ross D. A., Wilson C. B. Results of transsphenoidal microsurgery for growth hormone-secreting pituitary adenoma in a series of 214 patients. J Neurosurg. 1988 Jun;68(6):854–867. doi: 10.3171/jns.1988.68.6.0854. [DOI] [PubMed] [Google Scholar]
- Sano T., Asa S. L., Kovacs K. Growth hormone-releasing hormone-producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev. 1988 Aug;9(3):357–373. doi: 10.1210/edrv-9-3-357. [DOI] [PubMed] [Google Scholar]
- Scheithauer B. W., Kovacs K. T., Laws E. R., Jr, Randall R. V. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986 Dec;65(6):733–744. doi: 10.3171/jns.1986.65.6.0733. [DOI] [PubMed] [Google Scholar]
- Spada A., Elahi F. R., Arosio M., Sartorio A., Guglielmino L., Vallar L., Faglia G. Lack of desensitization of adenomatous somatotrophs to growth-hormone releasing hormone in acromegaly. J Clin Endocrinol Metab. 1987 Mar;64(3):585–591. doi: 10.1210/jcem-64-3-585. [DOI] [PubMed] [Google Scholar]
- Spada A., Vallar L., Faglia G. Cellular alterations in pituitary tumors. Eur J Endocrinol. 1994 Jan;130(1):43–52. doi: 10.1530/eje.0.1300043. [DOI] [PubMed] [Google Scholar]
- Stefaneanu L., Kovacs K., Horvath E., Asa S. L., Losinski N. E., Billestrup N., Price J., Vale W. Adenohypophysial changes in mice transgenic for human growth hormone-releasing factor: a histological, immunocytochemical, and electron microscopic investigation. Endocrinology. 1989 Nov;125(5):2710–2718. doi: 10.1210/endo-125-5-2710. [DOI] [PubMed] [Google Scholar]
- Stefaneanu L., Kovacs K., Horvath E., Lloyd R. V. In situ hybridization study of pro-opiomelanocortin (POMC) gene expression in human pituitary corticotrophs and their adenomas. Virchows Arch A Pathol Anat Histopathol. 1991;419(2):107–113. doi: 10.1007/BF01600224. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Shi S. R., Chaiwun B., Young L., Imam S. A., Cote R. J. Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol. 1994 Mar;25(3):263–270. doi: 10.1016/0046-8177(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Thapar K., Kovacs K., Horvath E., Stefaneanu L., Chambers E., Mortimer A. J. Effects of spaceflight on morphology of the rat adenohypophysis. J Appl Physiol (1985) 1994 Sep;77(3):1411–1420. doi: 10.1152/jappl.1994.77.3.1411. [DOI] [PubMed] [Google Scholar]
- Thapar K., Kovacs K., Laws E. R. The classification and molecular biology of pituitary adenomas. Adv Tech Stand Neurosurg. 1995;22:3–53. doi: 10.1007/978-3-7091-6898-1_1. [DOI] [PubMed] [Google Scholar]
- Thapar K., Kovacs K., Muller P. J. Clinical-pathological correlations of pituitary tumours. Baillieres Clin Endocrinol Metab. 1995 Apr;9(2):243–270. doi: 10.1016/s0950-351x(95)80322-x. [DOI] [PubMed] [Google Scholar]
- Thapar K., Stefaneanu L., Kovacs K., Scheithauer B. W., Lloyd R. V., Muller P. J., Laws E. R., Jr Estrogen receptor gene expression in craniopharyngiomas: an in situ hybridization study. Neurosurgery. 1994 Dec;35(6):1012–1017. doi: 10.1227/00006123-199412000-00002. [DOI] [PubMed] [Google Scholar]
- Vallar L., Spada A., Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987 Dec 10;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi I., Inokuchi K., Hasegawa O., Sugihara H., Minami S. Expression of growth hormone (GH)-releasing factor gene in GH-producing pituitary adenoma. J Clin Endocrinol Metab. 1992 Feb;74(2):357–361. doi: 10.1210/jcem.74.2.1730814. [DOI] [PubMed] [Google Scholar]
- Zarandi M., Horvath J. E., Halmos G., Pinski J., Nagy A., Groot K., Rekasi Z., Schally A. V. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]