Abstract
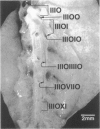
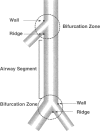
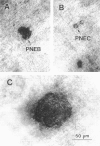
We report an immunohistochemical method for mapping the distribution of neuroepithelial bodies (NEBs) in whole-mount preparations of the intrapulmonary airways. The lungs of 8- and 50-day-old male Sprague-Dawley rats were fixed with ethanol-acetic acid by intratracheal instillation. The major axial airway path of the infracardiac lobe was exposed and isolated by microdissection. NEBs were identified by calcitonin gene-related peptide immunoreactivity and their distribution mapped by generation and branch-point number. A distinct pattern was noted with greater prevalence of NEBs in proximal airway generations compared with more distal airways. No significant difference was noted in the distribution pattern or absolute number of NEBs between neonates and adults when compared by airway generation. NEBs were found more frequently on the ridges of the bifurcation than in other regions of the bifurcating airway wall. The ease of identification of total numbers of NEBs and their specific location by airway generation in whole-mount preparations of the bronchial tree completely removes the necessity of examining multiple sections and performing extensive morphometric procedures. Whole-mount airway preparations allow for the analysis and comparison of larger sample sizes per experimental group without labor-intensive approaches. The application of this method should enhance our knowledge of the role of NEBs in lung development and in response to disease.
Full text
PDF


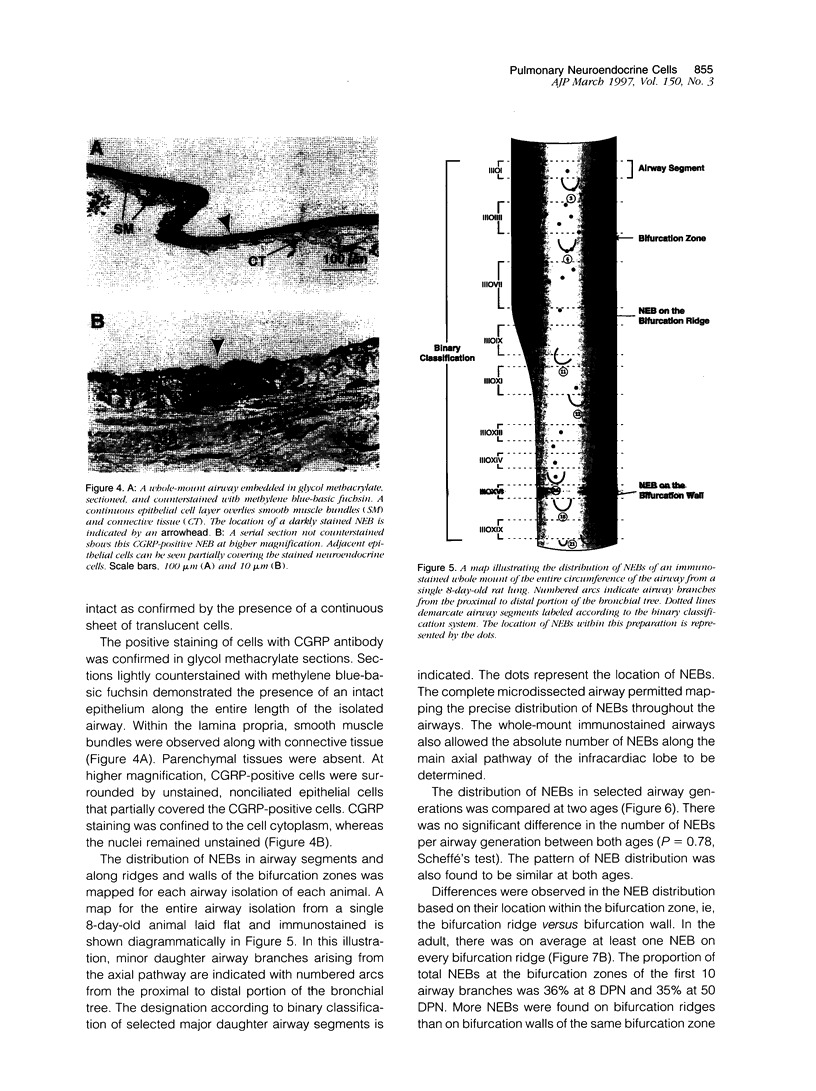
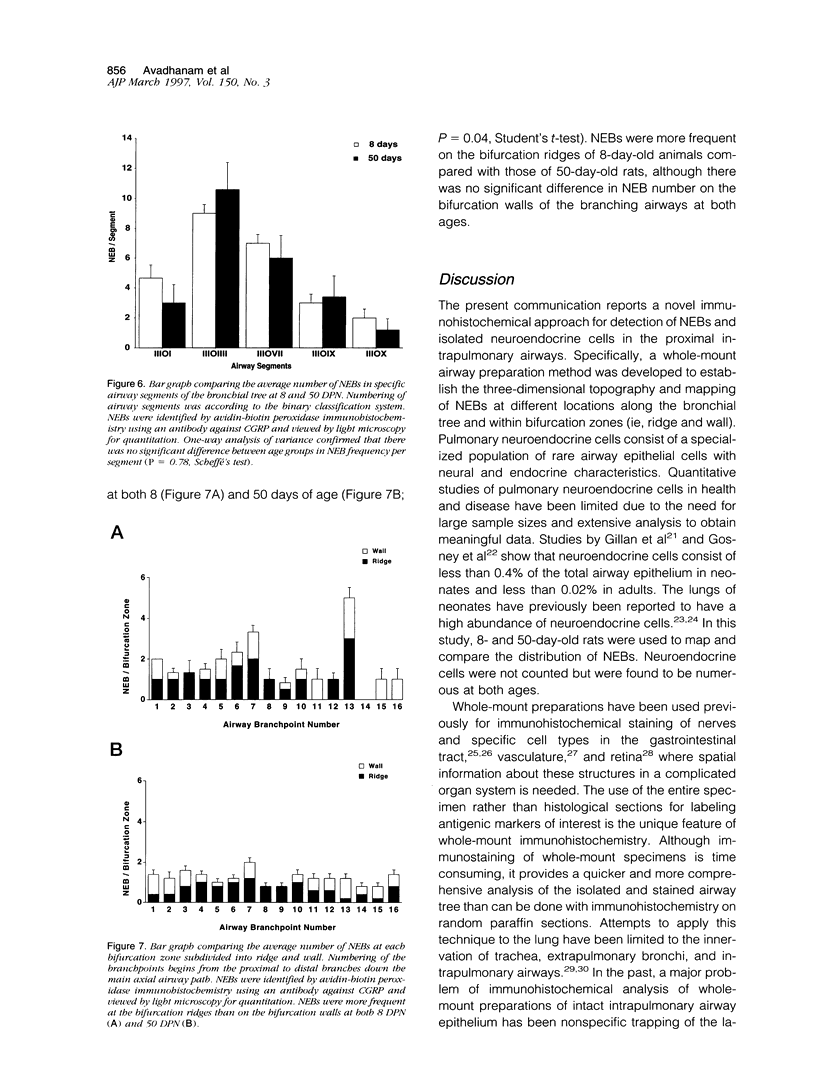



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burri P. H. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974 Sep;180(1):77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Cadieux A., Springall D. R., Mulderry P. K., Rodrigo J., Ghatei M. A., Terenghi G., Bloom S. R., Polak J. M. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: effect of capsaicin treatment and surgical denervations. Neuroscience. 1986 Oct;19(2):605–627. doi: 10.1016/0306-4522(86)90285-x. [DOI] [PubMed] [Google Scholar]
- Carabba V. H., Sorokin S. P., Hoyt R. F., Jr Development of neuroepithelial bodies in intact and cultured lungs of fetal rats. Am J Anat. 1985 May;173(1):1–27. doi: 10.1002/aja.1001730102. [DOI] [PubMed] [Google Scholar]
- Cho T., Chan W., Cutz E. Distribution and frequency of neuro-epithelial bodies in post-natal rabbit lung: quantitative study with monoclonal antibody against serotonin. Cell Tissue Res. 1989 Feb;255(2):353–362. doi: 10.1007/BF00224118. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Sonstegard K. S. Identification of neuro-epithelial bodies in rabbit fetal lungs by scanning electron microscopy: a correlative light, transmission and scanning electron microscopic study. Anat Rec. 1978 Nov;192(3):459–466. doi: 10.1002/ar.1091920311. [DOI] [PubMed] [Google Scholar]
- DiAugustine R. P., Sonstegard K. S. Neuroendocrinelike (small granule) epithelial cells of the lung. Environ Health Perspect. 1984 Apr;55:271–295. doi: 10.1289/ehp.8455271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan J. E., Pape K. E., Cutz E. Association of changes in bombesin immunoreactive neuroendocrine cells in lungs of newborn infants with persistent fetal circulation and brainstem damage due to birth asphyxia. Pediatr Res. 1986 Sep;20(9):828–833. doi: 10.1203/00006450-198609000-00003. [DOI] [PubMed] [Google Scholar]
- Gosney J. R., Sissons M. C., Allibone R. O. Neuroendocrine cell populations in normal human lungs: a quantitative study. Thorax. 1988 Nov;43(11):878–882. doi: 10.1136/thx.43.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage E., Hage J., Juel G. Endocrine-like cells of the pulmonary epithelium of the human adult lung. Cell Tissue Res. 1977 Mar 1;178(1):39–48. doi: 10.1007/BF00232822. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Feldman H., Sorokin S. P. Neuroepithelial bodies (NEB) and solitary endocrine cells in the hamster lung. Exp Lung Res. 1982 Nov;3(3-4):299–311. doi: 10.3109/01902148209069659. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, McNelly N. A., McDowell E. M., Sorokin S. P. Neuroepithelial bodies stimulate proliferation of airway epithelium in fetal hamster lung. Am J Physiol. 1991 Apr;260(4 Pt 1):L234–L240. doi: 10.1152/ajplung.1991.260.4.L234. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, McNelly N. A., Sorokin S. P. Dynamics of neuroepithelial body (NEB) formation in developing hamster lung: light microscopic autoradiography after 3H-thymidine labeling in vivo. Anat Rec. 1990 Jul;227(3):340–350. doi: 10.1002/ar.1092270309. [DOI] [PubMed] [Google Scholar]
- Hoyt R. F., Jr, Sorokin S. P., Feldman H. Small-granule (neuro)endocrine cells in the infracardiac lobe of a hamster lung. Number, subtypes, and distribution. Exp Lung Res. 1982 Nov;3(3-4):273–298. doi: 10.3109/01902148209069658. [DOI] [PubMed] [Google Scholar]
- Joad J. P., Ji C., Kott K. S., Bric J. M., Pinkerton K. E. In utero and postnatal effects of sidestream cigarette smoke exposure on lung function, hyperresponsiveness, and neuroendocrine cells in rats. Toxicol Appl Pharmacol. 1995 May;132(1):63–71. doi: 10.1006/taap.1995.1087. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Kulik T. J., Lock J. E., Elde R. P., Thompson T. R. Bombesin-, calcitonin-, and serotonin-immunoreactive pulmonary neuroendocrine cells in acute and chronic neonatal lung disease. Pediatr Pulmonol. 1985 May-Jun;1(3 Suppl):S13–S20. [PubMed] [Google Scholar]
- Johnson D. E., Lock J. E., Elde R. P., Thompson T. R. Pulmonary neuroendocrine cells in hyaline membrane disease and bronchopulmonary dysplasia. Pediatr Res. 1982 Jun;16(6):446–454. doi: 10.1203/00006450-198206000-00009. [DOI] [PubMed] [Google Scholar]
- King K. A., Torday J. S., Sunday M. E. Bombesin and [Leu8]phyllolitorin promote fetal mouse lung branching morphogenesis via a receptor-mediated mechanism. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4357–4361. doi: 10.1073/pnas.92.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding-Köppel M., Thier P., Koehler W. Morphology and mosaics of VIP-like immunoreactive neurons in the retina of the rhesus monkey. J Comp Neurol. 1991 Oct 8;312(2):251–263. doi: 10.1002/cne.903120208. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere M. Intrapulmonary neuro-epithelial bodies: hypoxia-sensitive neuro(chemo-)receptors. Experientia. 1973 Nov 15;29(11):1384–1386. doi: 10.1007/BF01922833. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Peuskens J. C. Neuro-epithelial bodies (neuroreceptor or secretory organs?) in human infant bronchial and bronchiolar epithelium. Anat Rec. 1972 Mar;172(3):471–481. doi: 10.1002/ar.1091720301. [DOI] [PubMed] [Google Scholar]
- Lippmann M., Schlesinger R. B. Interspecies comparisons of particle deposition and mucociliary clearance in tracheobronchial airways. J Toxicol Environ Health. 1984;13(2-3):441–469. doi: 10.1080/15287398409530509. [DOI] [PubMed] [Google Scholar]
- Maynard K. I., Ogilvy C. S. Patterns of peptide-containing perivascular nerves in the circle of Willis: their absence in intracranial arteriovenous malformations. J Neurosurg. 1995 May;82(5):829–833. doi: 10.3171/jns.1995.82.5.0829. [DOI] [PubMed] [Google Scholar]
- Nakatani Y. Pulmonary endocrine cells in infancy and childhood. Pediatr Pathol. 1991 Jan-Feb;11(1):31–48. doi: 10.3109/15513819109064740. [DOI] [PubMed] [Google Scholar]
- Pearson G. T. Structural organization and neuropeptide distributions in the equine enteric nervous system: an immunohistochemical study using whole-mount preparations from the small intestine. Cell Tissue Res. 1994 Jun;276(3):523–534. doi: 10.1007/BF00343949. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Becker K. L., Cutz E., Gail D. B., Goniakowska-Witalinska L., Gosney J. R., Lauweryns J. M., Linnoila I., McDowell E. M., Miller Y. E. Lung endocrine cell markers, peptides, and amines. Anat Rec. 1993 May;236(1):169–171. doi: 10.1002/ar.1092360120. [DOI] [PubMed] [Google Scholar]
- Redick M. L., Hung K. S. Quantitation of pulmonary neuroepithelial bodies in pre- and postnatal rabbits. Cell Tissue Res. 1984;238(3):583–587. doi: 10.1007/BF00219875. [DOI] [PubMed] [Google Scholar]
- Sarikas S. N., Hoyt R. F., Jr, Sorokin S. P. Small-granule APUD cells in relation to airway branching and growth: a quantitative, cartographic study in Syrian golden hamsters. Anat Rec. 1985 Nov;213(3):410–420. doi: 10.1002/ar.1092130307. [DOI] [PubMed] [Google Scholar]
- Skofitsch G., Jacobowitz D. M. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides. 1985 Jul-Aug;6(4):721–745. doi: 10.1016/0196-9781(85)90178-0. [DOI] [PubMed] [Google Scholar]
- Sonstegard K., Wong V., Cutz E. Neuro-epithelial bodies in organ cultures of fetal rabbit lungs. Ultrastructural characteristics and effects of drugs. Cell Tissue Res. 1979 Jun 8;199(1):159–170. doi: 10.1007/BF00237736. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Hoyt R. F., Jr Development of neuroepithelial bodies and solitary endocrine cells in fetal rabbit lungs. II. Nonspecific esterase as an indicator of early maturation. Exp Lung Res. 1982 Nov;3(3-4):261–272. doi: 10.3109/01902148209069657. [DOI] [PubMed] [Google Scholar]
- Sparrow M. P., Warwick S. P., Everett A. W. Innervation and function of the distal airways in the developing bronchial tree of fetal pig lung. Am J Respir Cell Mol Biol. 1995 Nov;13(5):518–525. doi: 10.1165/ajrcmb.13.5.7576686. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Hua J., Reyes B., Masui H., Torday J. S. Anti-bombesin monoclonal antibodies modulate fetal mouse lung growth and maturation in utero and in organ cultures. Anat Rec. 1993 May;236(1):25–34. doi: 10.1002/ar.1092360107. [DOI] [PubMed] [Google Scholar]
- Sunday M. E., Kaplan L. M., Motoyama E., Chin W. W., Spindel E. R. Gastrin-releasing peptide (mammalian bombesin) gene expression in health and disease. Lab Invest. 1988 Jul;59(1):5–24. [PubMed] [Google Scholar]
- Tateishi R. Distribution of argyrophil cells in adult human lungs. Arch Pathol. 1973 Sep;96(3):198–202. [PubMed] [Google Scholar]
- Tsutsumi Y., Osamura R. Y., Watanabe K., Yanaihara N. Immunohistochemical studies on gastrin-releasing peptide- and adrenocorticotropic hormone-containing cells in the human lung. Lab Invest. 1983 May;48(5):623–632. [PubMed] [Google Scholar]
- Tsutsumi Y., Osamura R. Y., Watanabe K., Yanaihara N. Simultaneous immunohistochemical localization of gastrin releasing peptide (GRP) and calcitonin (CT) in human bronchial endocrine-type cells. Virchows Arch A Pathol Anat Histopathol. 1983;400(2):163–171. doi: 10.1007/BF00585498. [DOI] [PubMed] [Google Scholar]
- Wells D. G., Talmage E. K., Mawe G. M. Immunohistochemical identification of neurons in ganglia of the guinea pig sphincter of Oddi. J Comp Neurol. 1995 Jan 30;352(1):106–116. doi: 10.1002/cne.903520108. [DOI] [PubMed] [Google Scholar]
- Youngson C., Nurse C., Yeger H., Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993 Sep 9;365(6442):153–155. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]
- Zeltner T. B., Caduff J. H., Gehr P., Pfenninger J., Burri P. H. The postnatal development and growth of the human lung. I. Morphometry. Respir Physiol. 1987 Mar;67(3):247–267. doi: 10.1016/0034-5687(87)90057-0. [DOI] [PubMed] [Google Scholar]