Abstract
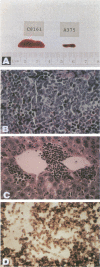
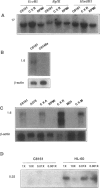
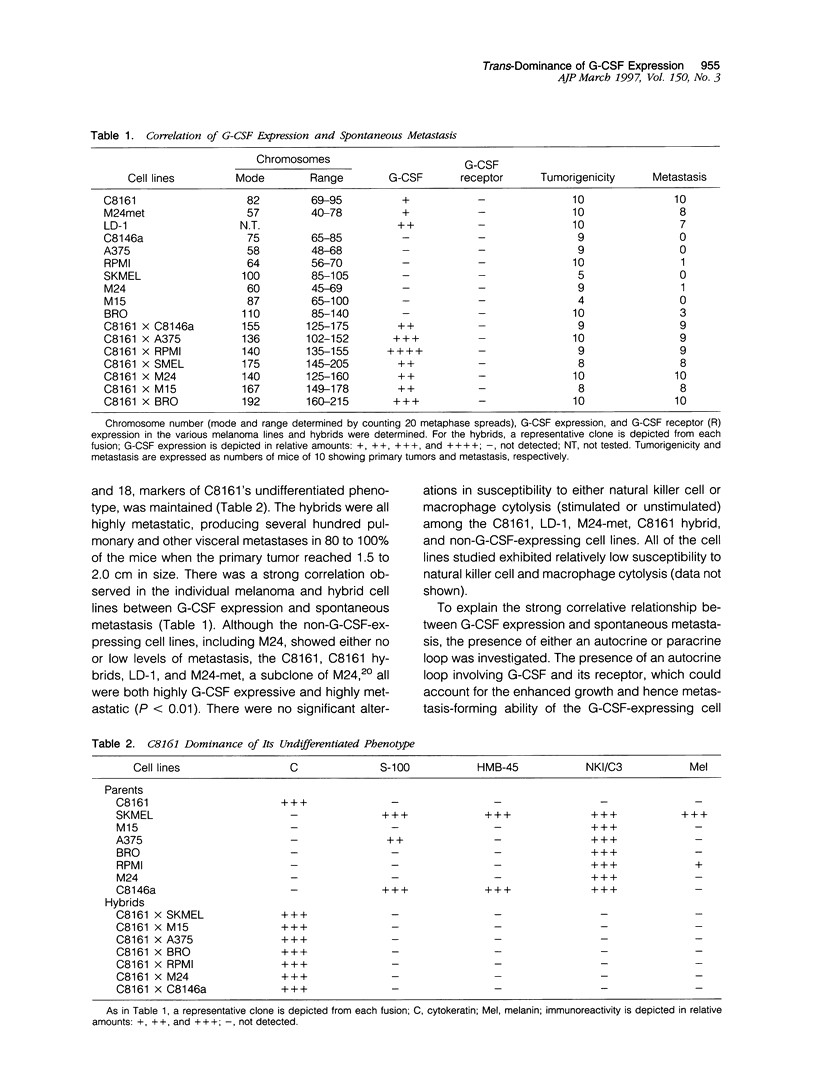
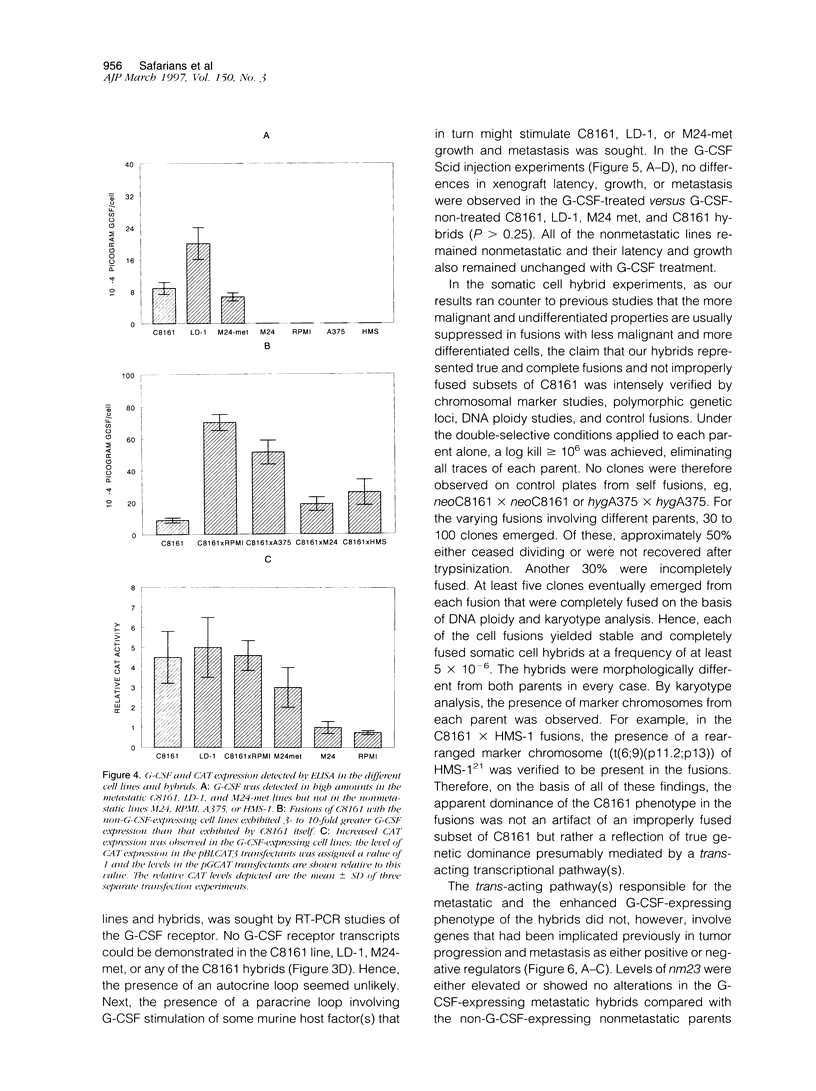
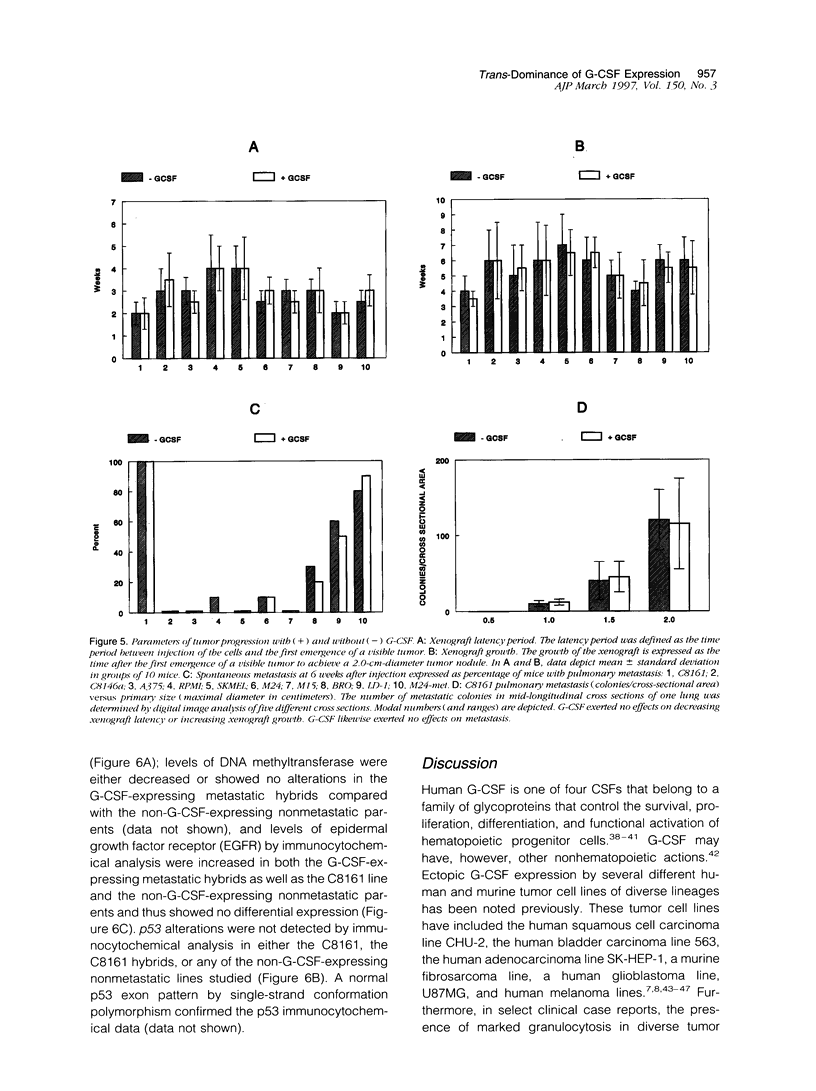
Using a human melanoma/Scid xenograft model with the C8161, M24-met, LD-1 and other human melanoma lines to investigate spontaneous metastasis, we made the observation of marked splenomegaly (up to five times normal weight and size) in only those xenografts exhibiting high degrees of spontaneous metastasis. Evaluation of this revealed the cause to be massive myelopoiesis due to ectopic granulocyte/ colony-stimulating factor (G-CSF) production by the melanoma cells. Because of these observations linking G-CSF expression with metastasis of human melanoma, we decided to investigate the mechanism of this ectopic production. No gross amplification or rearrangement of the G-CSF gene could be detected as the basis for the increased transcriptional activity in any of these lines. Human-human somatic cell hybridization studies carried out between the metastatic C8161 and several different nonmetastatic non-G-CSF-expressing lines revealed, in addition to metastatic dominance, 3- to 10-fold enhancement of G-CSF transcription and expression in the fusions compared with C8161 itself. The suggestion of a trans-dominant mechanism was further supported by transfection studies with a human G-CSF promoter-CAT-reporter construct, which revealed 3- to 5-fold increased reporter activity in only those melanoma lines and hybrids expressing G-CSF. Furthermore, no obvious autocrine or paracrine effects of this ectopic G-CSF expression on the melanoma lines' growth or metastasis were apparent, as all of the G-CSF-expressing lines lacked the G-CSF receptor and injections of purified recombinant G-CSF exerted no stimulatory effects on their tumorigenicity, latency, growth, or metastasis in Scid mice. Thus, we advance the hypothesis that G-CSF expression is serving as a marker of a more generalized trans-dominant pathway linked to tumor progression and metastasis. This hypothesis has direct relevance to many human cancers where ectopic hormone or growth factor production occurs with no obvious autocrine or paracrine benefit to the tumor.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berdel W. E., Danhauser-Riedl S., Steinhauser G., Winton E. F. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989 Jan;73(1):80–83. [PubMed] [Google Scholar]
- Berdel W. E., Oberberg D., Reufi B., Thiel E. Studies on the role of recombinant human erythropoietin in the growth regulation of human nonhematopoietic tumor cells in vitro. Ann Hematol. 1991 Jul;63(1):5–8. doi: 10.1007/BF01714953. [DOI] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cross M., Dexter T. M. Growth factors in development, transformation, and tumorigenesis. Cell. 1991 Jan 25;64(2):271–280. doi: 10.1016/0092-8674(91)90638-f. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Fu Y. S., Cheng L., Huang I., Huang S., Wiesmeier E., Wettstein F., Weissman M. DNA ploidy analysis of cervical condyloma and intraepithelial neoplasia in specimens obtained by punch biopsy. Anal Quant Cytol Histol. 1989 Jun;11(3):187–195. [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Janik J. E., Sznol M., Urba W. J., Figlin R., Bukowski R. M., Fyfe G., Pierce W. C., Belldegrun A., Sharfman W. H., Smith J. W., 2nd Erythropoietin production. A potential marker for interleukin-2/interferon-responsive tumors. Cancer. 1993 Nov 1;72(9):2656–2659. doi: 10.1002/1097-0142(19931101)72:9<2656::aid-cncr2820720922>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Jones P. A. DNA methylation and cancer. Cancer Res. 1986 Feb;46(2):461–466. [PubMed] [Google Scholar]
- Kaighn M. E., Gabrielson E. W., Iman D. S., Pauls E. A., Harris C. C. Suppression of tumorigenicity of a human lung carcinoma line by nontumorigenic bronchial epithelial cells in somatic cell hybrids. Cancer Res. 1990 Mar 15;50(6):1890–1896. [PubMed] [Google Scholar]
- Kozlowski J. M., Fidler I. J., Campbell D., Xu Z. L., Kaighn M. E., Hart I. R. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984 Aug;44(8):3522–3529. [PubMed] [Google Scholar]
- Leone A., Flatow U., King C. R., Sandeen M. A., Margulies I. M., Liotta L. A., Steeg P. S. Reduced tumor incidence, metastatic potential, and cytokine responsiveness of nm23-transfected melanoma cells. Cell. 1991 Apr 5;65(1):25–35. doi: 10.1016/0092-8674(91)90404-m. [DOI] [PubMed] [Google Scholar]
- Lilly M. B., Devlin P. E., Devlin J. J., Rado T. A. Production of granulocyte colony-stimulating factor by a human melanoma cell line. Exp Hematol. 1987 Oct;15(9):966–971. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Lockshin A., Giovanella B. C., De Ipolyi P. D., Williams L. J., Jr, Mendoza J. T., Yim S. O., Stehlin J. S., Jr Exceptional lethality for nude mice of cells derived from a primary human melanoma. Cancer Res. 1985 Jan;45(1):345–350. [PubMed] [Google Scholar]
- Lokich J. J. The frequency and clinical biology of the ectopic hormone syndromes of small cell carcinoma. Cancer. 1982 Nov 15;50(10):2111–2114. doi: 10.1002/1097-0142(19821115)50:10<2111::aid-cncr2820501023>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Scheinman S. J. Ectopic secretion of neurohypophyseal peptides in patients with malignancy. Endocrinol Metab Clin North Am. 1991 Sep;20(3):489–506. [PubMed] [Google Scholar]
- Mueller B. M., Romerdahl C. A., Trent J. M., Reisfeld R. A. Suppression of spontaneous melanoma metastasis in scid mice with an antibody to the epidermal growth factor receptor. Cancer Res. 1991 Apr 15;51(8):2193–2198. [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Tsuchiya M., Watanabe-Fukunaga R., Nagata S. Multiple elements in the promoter of granulocyte colony-stimulating factor gene regulate its constitutive expression in human carcinoma cells. J Biol Chem. 1990 Apr 5;265(10):5897–5902. [PubMed] [Google Scholar]
- Nomura H., Imazeki I., Oheda M., Kubota N., Tamura M., Ono M., Ueyama Y., Asano S. Purification and characterization of human granulocyte colony-stimulating factor (G-CSF). EMBO J. 1986 May;5(5):871–876. doi: 10.1002/j.1460-2075.1986.tb04297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff J. J., Kats Y., Urena P., Schipani E., Vasavada R. C., Philbrick W. M., Behal A., Abou-Samra A. B., Segre G. V., Jüppner H. Further evidence for a novel receptor for amino-terminal parathyroid hormone-related protein on keratinocytes and squamous carcinoma cell lines. Endocrinology. 1995 Jul;136(7):3016–3023. doi: 10.1210/endo.136.7.7789327. [DOI] [PubMed] [Google Scholar]
- Pekarek L. A., Starr B. A., Toledano A. Y., Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995 Jan 1;181(1):435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan D. C., Davidson A. G., Summers C. L., Warden H. E., Doshi H. M. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992 Sep 1;52(17):4828–4831. [PubMed] [Google Scholar]
- Ramshaw I. A., Carlsen S., Wang H. C., Badenoch-Jones P. The use of cell fusion to analyse factors involved in tumour cell metastasis. Int J Cancer. 1983 Oct 15;32(4):471–478. doi: 10.1002/ijc.2910320414. [DOI] [PubMed] [Google Scholar]
- Safarians S., Sternlicht M. D., Freiman C. J., Huaman J. A., Barsky S. H. The primary tumor is the primary source of metastasis in a human melanoma/SCID model. Implications for the direct autocrine and paracrine epigenetic regulation of the metastasis process. Int J Cancer. 1996 Apr 10;66(2):151–158. doi: 10.1002/(SICI)1097-0215(19960410)66:2<151::AID-IJC2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Safarians S., Sternlicht M. D., Yamanishi D. T., Love S. M., Barsky S. H. Human breast cancer progression can be regulated by dominant trans-acting factors in somatic cell hybridization studies. Cancer Res. 1996 Aug 1;56(15):3560–3569. [PubMed] [Google Scholar]
- Schadendorf D., Möller A., Algermissen B., Worm M., Sticherling M., Czarnetzki B. M. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993 Sep 1;151(5):2667–2675. [PubMed] [Google Scholar]
- Shannon M. F., Coles L. S., Fielke R. K., Goodall G. J., Lagnado C. A., Vadas M. A. Three essential promoter elements mediate tumour necrosis factor and interleukin-1 activation of the granulocyte-colony stimulating factor gene. Growth Factors. 1992;7(3):181–193. doi: 10.3109/08977199209046923. [DOI] [PubMed] [Google Scholar]
- Sharma R. C., Murphy A. J., DeWald M. G., Schimke R. T. A rapid procedure for isolation of RNA-free genomic DNA from mammalian cells. Biotechniques. 1993 Feb;14(2):176–178. [PubMed] [Google Scholar]
- Shih I. M., Herlyn M. Role of growth factors and their receptors in the development and progression of melanoma. J Invest Dermatol. 1993 Feb;100(2 Suppl):196S–203S. [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Rideout W. M., 3rd, Olumi A. F., Ohneseit P. F., Yang A. S., Tsai Y. C., Nichols P. W., Horn T., Hermann G. G., Steven K. Distinct pattern of p53 mutations in bladder cancer: relationship to tobacco usage. Cancer Res. 1993 Mar 1;53(5):1162–1166. [PubMed] [Google Scholar]
- Stan A. C., Walter G. F., Welte K., Pietsch T. Immunolocalization of granulocyte-colony-stimulating factor in human glial and primitive neuroectodermal tumors. Int J Cancer. 1994 May 1;57(3):306–312. doi: 10.1002/ijc.2910570303. [DOI] [PubMed] [Google Scholar]
- Staroselsky A. H., Pathak S., Chernajovsky Y., Tucker S. L., Fidler I. J. Predominance of the metastatic phenotype in somatic cell hybrids of the K-1735 murine melanoma. Cancer Res. 1991 Dec 1;51(23 Pt 1):6292–6298. [PubMed] [Google Scholar]
- Steeg P. S., Bevilacqua G., Kopper L., Thorgeirsson U. P., Talmadge J. E., Liotta L. A., Sobel M. E. Evidence for a novel gene associated with low tumor metastatic potential. J Natl Cancer Inst. 1988 Apr 6;80(3):200–204. doi: 10.1093/jnci/80.3.200. [DOI] [PubMed] [Google Scholar]
- Sternlicht M. D., Safarians S., Rivera S. P., Barsky S. H. Characterizations of the extracellular matrix and proteinase inhibitor content of human myoepithelial tumors. Lab Invest. 1996 Apr;74(4):781–796. [PubMed] [Google Scholar]
- Tait D. L., MacDonald P. C., Casey M. L. Parathyroid hormone-related protein expression in gynecic squamous carcinoma cells. Cancer. 1994 Mar 1;73(5):1515–1521. doi: 10.1002/1097-0142(19940301)73:5<1515::aid-cncr2820730532>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Isobe T., Ohtsuki Y., Akagi T., Sonobe H., Okuyama T. Immunohistochemical study on the distribution of alpha and beta subunits of S-100 protein in human neoplasm and normal tissues. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):385–396. doi: 10.1007/BF02889881. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Wada T., Mori M., Kokai Y., Ishii S. Overexpression of the granulocyte colony-stimulating factor gene leads to osteoporosis in mice. Lab Invest. 1996 Apr;74(4):827–834. [PubMed] [Google Scholar]
- Tsuchida T., Saxton R. E., Irie R. F. Gangliosides of human melanoma: GM2 and tumorigenicity. J Natl Cancer Inst. 1987 Jan;78(1):55–60. doi: 10.1093/jnci/78.1.55. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M., Asano S., Kaziro Y., Nagata S. Isolation and characterization of the cDNA for murine granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7633–7637. doi: 10.1073/pnas.83.20.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A. M., Zsebo K. M., Martin F., Jacobsen F. W., Bennett L. G., Broudy V. C. Nonhematopoietic tumor cell lines express stem cell factor and display c-kit receptors. Blood. 1992 Jul 15;80(2):374–381. [PubMed] [Google Scholar]
- Tweardy D. J., Anderson K., Cannizzaro L. A., Steinman R. A., Croce C. M., Huebner K. Molecular cloning of cDNAs for the human granulocyte colony-stimulating factor receptor from HL-60 and mapping of the gene to chromosome region 1p32-34. Blood. 1992 Mar 1;79(5):1148–1154. [PubMed] [Google Scholar]
- Tweardy D. J., Cannizzaro L. A., Palumbo A. P., Shane S., Huebner K., Vantuinen P., Ledbetter D. H., Finan J. B., Nowell P. C., Rovera G. Molecular cloning and characterization of a cDNA for human granulocyte colony-stimulating factor (G-CSF) from a glioblastoma multiforme cell line and localization of the G-CSF gene to chromosome band 17q21. Oncogene Res. 1987 Aug;1(3):209–220. [PubMed] [Google Scholar]
- Vennegoor C., Calafat J., Hageman P., van Buitenen F., Janssen H., Kolk A., Rümke P. Biochemical characterization and cellular localization of a formalin-resistant melanoma-associated antigen reacting with monoclonal antibody NKI/C-3. Int J Cancer. 1985 Mar 15;35(3):287–295. doi: 10.1002/ijc.2910350302. [DOI] [PubMed] [Google Scholar]
- Welch D. R., Bisi J. E., Miller B. E., Conaway D., Seftor E. A., Yohem K. H., Gilmore L. B., Seftor R. E., Nakajima M., Hendrix M. J. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991 Jan 21;47(2):227–237. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Dexter T. M. Myeloid haemopoietic growth factors. Biochim Biophys Acta. 1989 Dec 17;989(2):111–132. doi: 10.1016/0304-419x(89)90038-3. [DOI] [PubMed] [Google Scholar]
- Yamanishi D. T., Buckmeier J. A., Meyskens F. L., Jr Expression of c-jun, jun-B, and c-fos proto-oncogenes in human primary melanocytes and metastatic melanomas. J Invest Dermatol. 1991 Aug;97(2):349–353. doi: 10.1111/1523-1747.ep12480698. [DOI] [PubMed] [Google Scholar]
- Yen R. W., Vertino P. M., Nelkin B. D., Yu J. J., el-Deiry W., Cumaraswamy A., Lennon G. G., Trask B. J., Celano P., Baylin S. B. Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 1992 May 11;20(9):2287–2291. doi: 10.1093/nar/20.9.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]