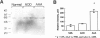Abstract
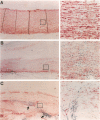
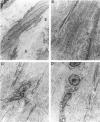
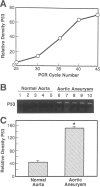
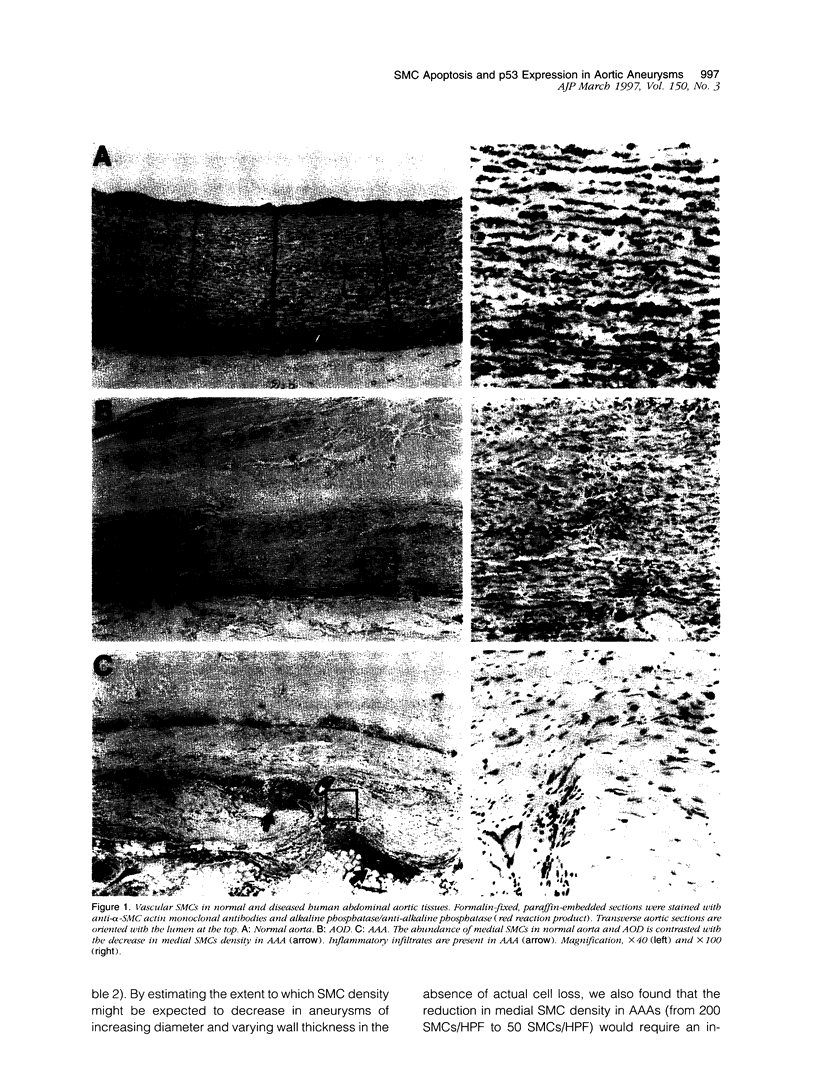
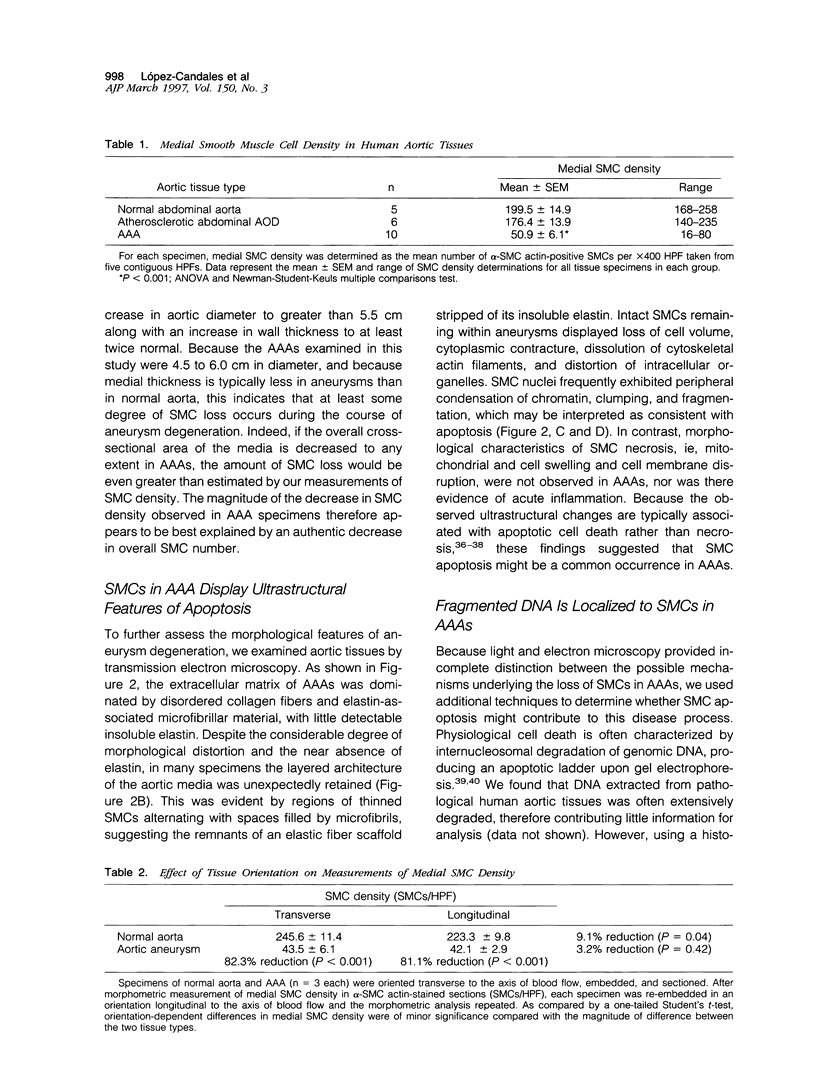
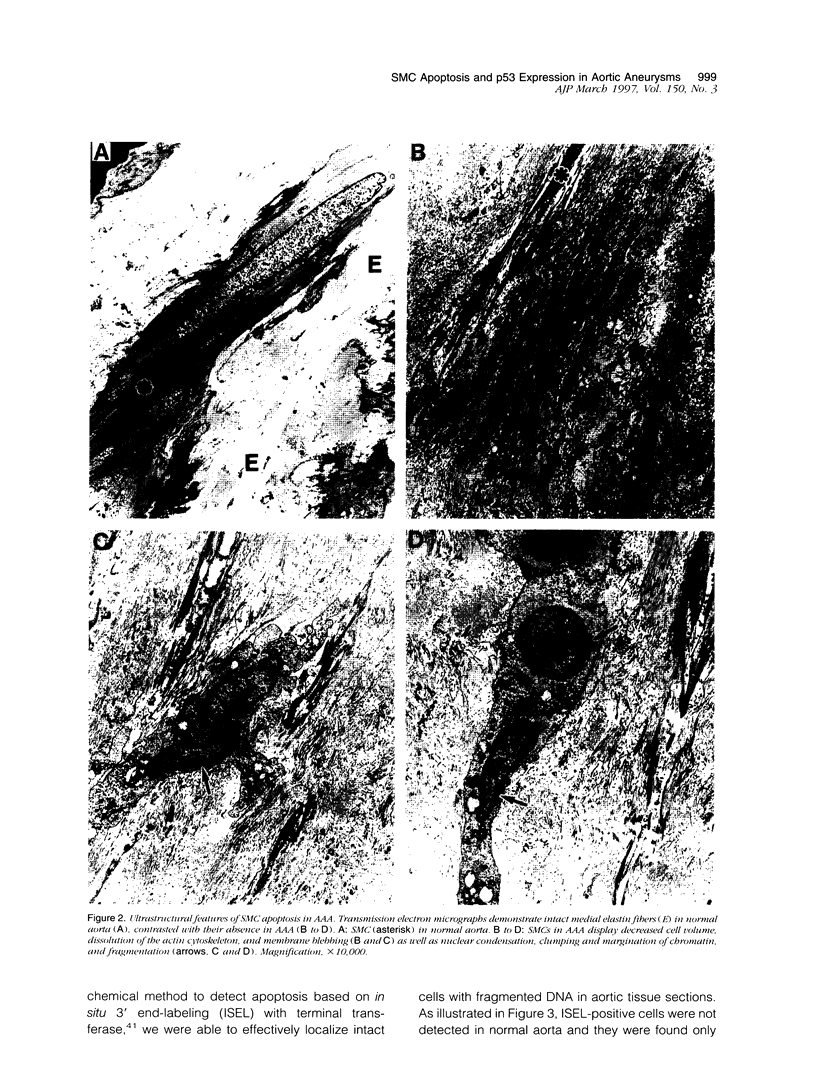
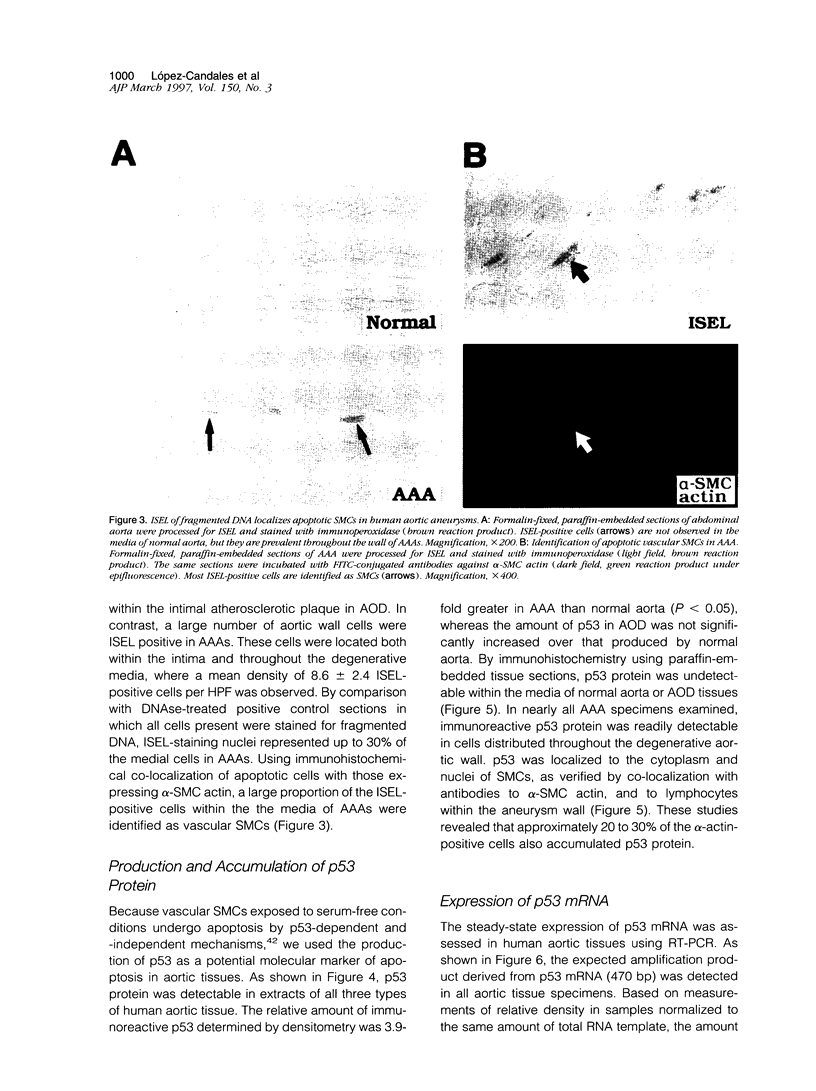
Abdominal aortic aneurysms (AAAs) are characterized by structural deterioration of the aortic wall leading to progressive aortic dilatation and eventual rupture. The histopathological changes in AAAs are particularly evident within the elastic media, which is normally dominated by vascular smooth muscle cells (SMCs). To determine whether a decrease in vascular SMCs contributes to medial degeneration, we measured SMC density in 21 normal and pathological human abdominal aortic tissue specimens using immunohistochemistry for alpha-SMC actin and direct cell counts (medial SMCs per high-power field (HPF)). Medial SMC density was not significantly different between normal aorta (n = 5; 199.5 +/- 14.9 SMCs/HPF) and atherosclerotic occlusive disease (n = 6; 176.4 +/- 13.9 SMCs/HPF), but it was reduced by 74% in AAA (n = 10; 50.9 +/- 6.1 SMCs/HPF; P < 0.01 versus normal aorta). Light and electron microscopy revealed no evidence of overt cellular necrosis, but SMCs in AAAs exhibited ultrastructural changes consistent with apoptosis. Using in situ end-labeling (ISEL) of fragmented DNA to detect apoptotic cells, up to 30% of aortic wall cells were ISEL positive in AAAs. By double-labeling techniques, many of these cells were alpha-actin-positive SMCs distributed throughout the degenerative media. In contrast, ISEL-positive cells were observed only within the intimal plaque in atherosclerotic occlusive disease. The amount of p53 protein detected by immunoblotting was increased nearly fourfold in AAA compared with normal aorta and atherosclerotic occlusive disease (P < 0.01), and immunoreactive p53 was localized to lymphocytes and residual SMCs in the aneurysm wall. Using reverse transcription polymerase chain reaction assays a substantial amount of p53 mRNA expression was observed in AAAs. These results demonstrate that medial SMC density is significantly decreased in human AAA tissues associated with evidence of SMC apoptosis and increased production of p53, a potential mediator of cell cycle arrest and programmed cell death. Given the role that SMCs normally play in maintaining medial architecture and in arterial wall matrix remodeling, the induction of SMC apoptosis likely makes an important contribution to the evolution of aneurysm degeneration.
Full text
PDF



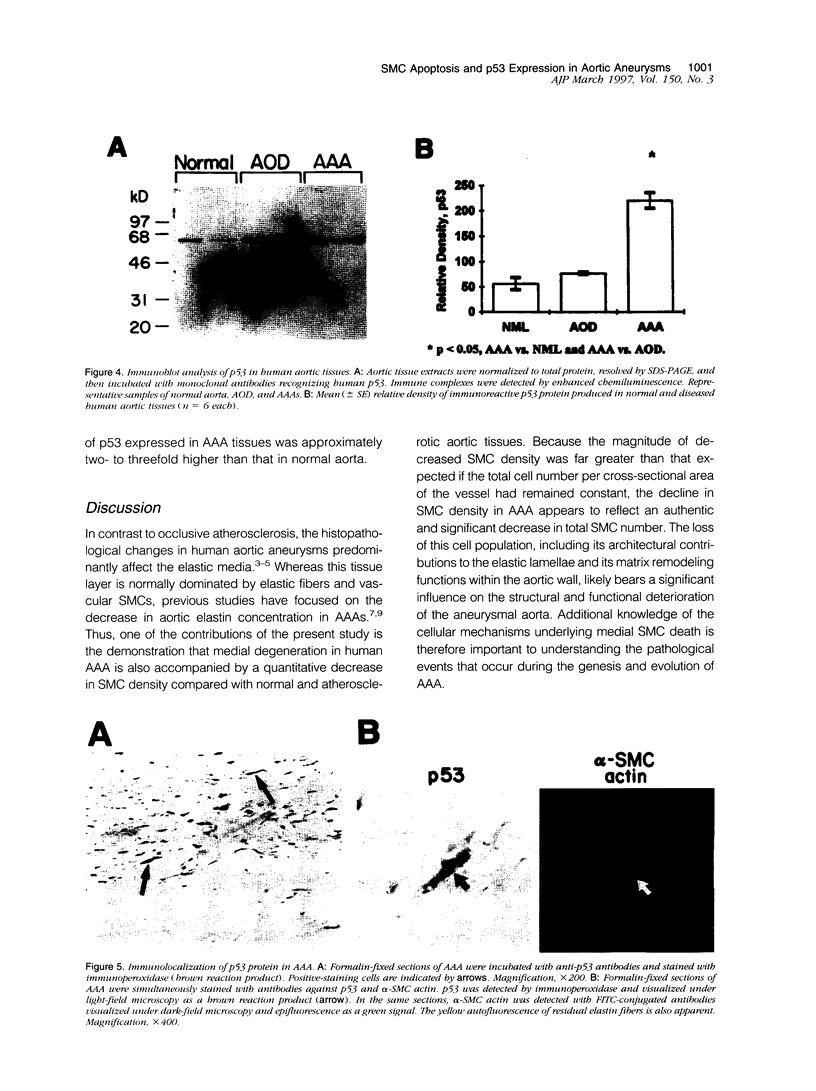
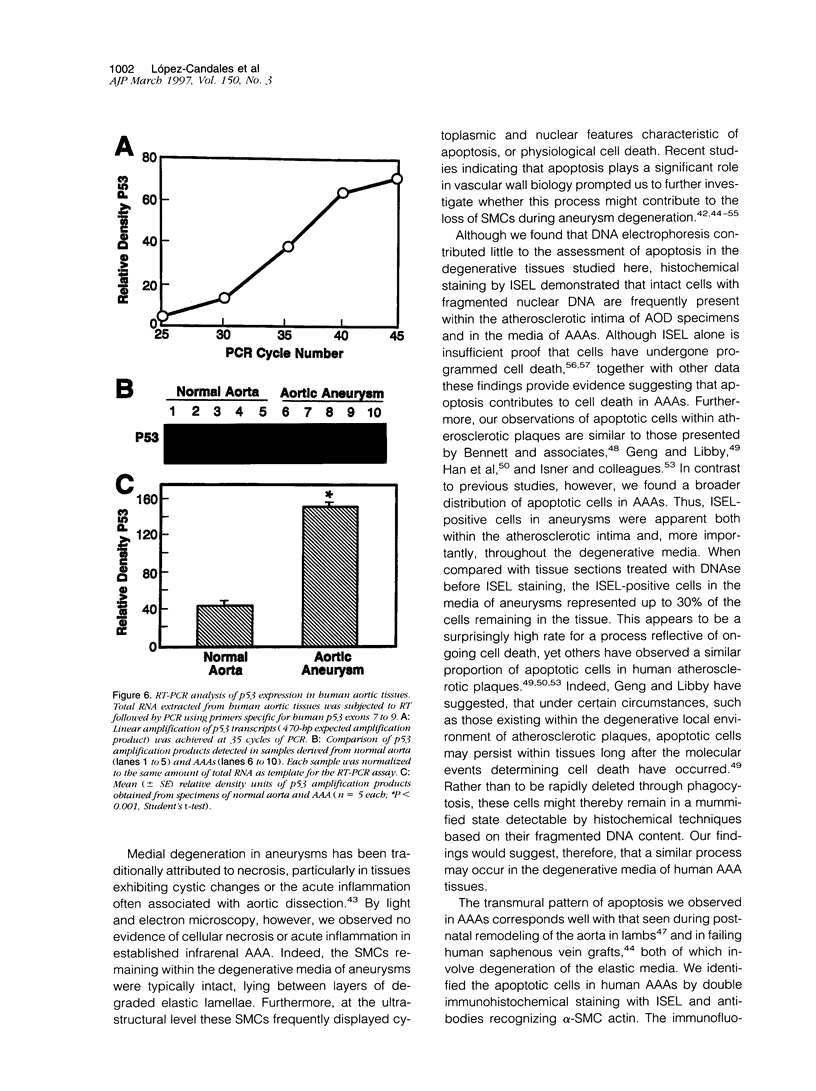





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anidjar S., Salzmann J. L., Gentric D., Lagneau P., Camilleri J. P., Michel J. B. Elastase-induced experimental aneurysms in rats. Circulation. 1990 Sep;82(3):973–981. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Barath P., Fishbein M. C., Cao J., Berenson J., Helfant R. H., Forrester J. S. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol. 1990 Feb 1;65(5):297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- Baxter B. T., McGee G. S., Shively V. P., Drummond I. A., Dixit S. N., Yamauchi M., Pearce W. H. Elastin content, cross-links, and mRNA in normal and aneurysmal human aorta. J Vasc Surg. 1992 Aug;16(2):192–200. [PubMed] [Google Scholar]
- Bendeck M. P., Zempo N., Clowes A. W., Galardy R. E., Reidy M. A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994 Sep;75(3):539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Newby A. C. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-gamma, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ Res. 1994 Mar;74(3):525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Schwartz S. M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995 May;95(5):2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Schwartz S. M. Apoptosis of rat vascular smooth muscle cells is regulated by p53-dependent and -independent pathways. Circ Res. 1995 Aug;77(2):266–273. doi: 10.1161/01.res.77.2.266. [DOI] [PubMed] [Google Scholar]
- Björkerud B., Björkerud S. Contrary effects of lightly and strongly oxidized LDL with potent promotion of growth versus apoptosis on arterial smooth muscle cells, macrophages, and fibroblasts. Arterioscler Thromb Vasc Biol. 1996 Mar;16(3):416–424. doi: 10.1161/01.atv.16.3.416. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat M. L., Gabbiani F., Redard M., Desmoulière A., Gabbiani G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am J Pathol. 1995 May;146(5):1059–1064. [PMC free article] [PubMed] [Google Scholar]
- Brophy C. M., Reilly J. M., Smith G. J., Tilson M. D. The role of inflammation in nonspecific abdominal aortic aneurysm disease. Ann Vasc Surg. 1991 May;5(3):229–233. doi: 10.1007/BF02329378. [DOI] [PubMed] [Google Scholar]
- Campa J. S., Greenhalgh R. M., Powell J. T. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987 May;65(1-2):13–21. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- Cho A., Courtman D. W., Langille B. L. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res. 1995 Feb;76(2):168–175. doi: 10.1161/01.res.76.2.168. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. R., Mandell C., Chang J. B., Wise L. Elastin metabolism of the infrarenal aorta. J Vasc Surg. 1988 Feb;7(2):210–214. doi: 10.1067/mva.1988.avs0070210. [DOI] [PubMed] [Google Scholar]
- Cohen J. R., Sarfati I., Danna D., Wise L. Smooth muscle cell elastase, atherosclerosis, and abdominal aortic aneurysms. Ann Surg. 1992 Sep;216(3):327–332. doi: 10.1097/00000658-199209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrin P. B., Baker W. H., Gley W. C. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch Surg. 1984 Apr;119(4):405–409. doi: 10.1001/archsurg.1984.01390160041009. [DOI] [PubMed] [Google Scholar]
- Dobrin P. B., Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994 Aug;2(4):484–488. [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Ernst C. B. Abdominal aortic aneurysm. N Engl J Med. 1993 Apr 22;328(16):1167–1172. doi: 10.1056/NEJM199304223281607. [DOI] [PubMed] [Google Scholar]
- Freestone T., Turner R. J., Coady A., Higman D. J., Greenhalgh R. M., Powell J. T. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995 Aug;15(8):1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- Gandhi R. H., Irizarry E., Cantor J. O., Keller S., Nackman G. B., Halpern V. J., Newman K. M., Tilson M. D. Analysis of elastin cross-linking and the connective tissue matrix of abdominal aortic aneurysms. Surgery. 1994 May;115(5):617–620. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995 Aug;147(2):251–266. [PMC free article] [PubMed] [Google Scholar]
- Geng Y. J., Wu Q., Muszynski M., Hansson G. K., Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler Thromb Vasc Biol. 1996 Jan;16(1):19–27. doi: 10.1161/01.atv.16.1.19. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B., Ruttkay-Nedecky B., Koudelka H., Bukowska K., Bursch W., Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995 May;21(5):1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Han D. K., Haudenschild C. C., Hong M. K., Tinkle B. T., Leon M. B., Liau G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am J Pathol. 1995 Aug;147(2):267–277. [PMC free article] [PubMed] [Google Scholar]
- He C. M., Roach M. R. The composition and mechanical properties of abdominal aortic aneurysms. J Vasc Surg. 1994 Jul;20(1):6–13. doi: 10.1016/0741-5214(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Herron G. S., Unemori E., Wong M., Rapp J. H., Hibbs M. H., Stoney R. J. Connective tissue proteinases and inhibitors in abdominal aortic aneurysms. Involvement of the vasa vasorum in the pathogenesis of aortic aneurysms. Arterioscler Thromb. 1991 Nov-Dec;11(6):1667–1677. doi: 10.1161/01.atv.11.6.1667. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. Defining apoptosis. Am J Pathol. 1995 Jan;146(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- Holifield B., Helgason T., Jemelka S., Taylor A., Navran S., Allen J., Seidel C. Differentiated vascular myocytes: are they involved in neointimal formation? J Clin Invest. 1996 Feb 1;97(3):814–825. doi: 10.1172/JCI118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Holmes D. R., Liao S., Parks W. C., Thompson R. W. Medial neovascularization in abdominal aortic aneurysms: a histopathologic marker of aneurysmal degeneration with pathophysiologic implications. J Vasc Surg. 1995 May;21(5):761–772. doi: 10.1016/s0741-5214(05)80007-2. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Kearney M., Bortman S., Passeri J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995 Jun 1;91(11):2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Foster C. L., Saunders W. B., Stang P. E. Social consequences of psychiatric disorders, I: Educational attainment. Am J Psychiatry. 1995 Jul;152(7):1026–1032. doi: 10.1176/ajp.152.7.1026. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Haines G. K., Rizzo R. J., Radosevich J. A., Pope R. M., Robinson P. G., Pearce W. H. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immune-mediated response. Am J Pathol. 1990 Nov;137(5):1199–1213. [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Pearce W. H., Shah M. R., Parikh D., Evanoff H. L., Haines G. K., Burdick M. D., Strieter R. M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993 May;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Kockx M. M., Cambier B. A., Bortier H. E., De Meyer G. R., Declercq S. C., van Cauwelaert P. A., Bultinck J. Foam cell replication and smooth muscle cell apoptosis in human saphenous vein grafts. Histopathology. 1994 Oct;25(4):365–371. doi: 10.1111/j.1365-2559.1994.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Romanic A. M., Prockop D. J. A mutation in the gene for type III procollagen (COL3A1) in a family with aortic aneurysms. J Clin Invest. 1990 Nov;86(5):1465–1473. doi: 10.1172/JCI114863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Petit P., Zamzami N., Vayssière J. L., Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995 Oct;9(13):1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Lefevre M., Rucker R. B. Aorta elastin turnover in normal and hypercholesterolemic Japanese quail. Biochim Biophys Acta. 1980 Jul 15;630(4):519–529. doi: 10.1016/0304-4165(80)90006-9. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- McKusick V. A. The defect in Marfan syndrome. Nature. 1991 Jul 25;352(6333):279–281. doi: 10.1038/352279a0. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundle S. D., Raza A. The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis. Lab Invest. 1995 May;72(5):611–613. [PubMed] [Google Scholar]
- Newman K. M., Malon A. M., Shin R. D., Scholes J. V., Ramey W. G., Tilson M. D. Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect Tissue Res. 1994;30(4):265–276. doi: 10.3109/03008209409015042. [DOI] [PubMed] [Google Scholar]
- Okada Y., Katsuda S., Okada Y., Nakanishi I. An elastinolytic enzyme detected in the culture medium of human arterial smooth muscle cells. Cell Biol Int. 1993 Sep;17(9):863–869. doi: 10.1006/cbir.1993.1149. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce W. H., Sweis I., Yao J. S., McCarthy W. J., Koch A. E. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992 Nov;16(5):784–789. [PubMed] [Google Scholar]
- Pollman M. J., Yamada T., Horiuchi M., Gibbons G. H. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ Res. 1996 Oct;79(4):748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- Powell J., Greenhalgh R. M. Cellular, enzymatic, and genetic factors in the pathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1989 Feb;9(2):297–304. doi: 10.1067/mva.1989.vs0090297. [DOI] [PubMed] [Google Scholar]
- Rasmussen L. M., Wolf Y. G., Ruoslahti E. Vascular smooth muscle cells from injured rat aortas display elevated matrix production associated with transforming growth factor-beta activity. Am J Pathol. 1995 Oct;147(4):1041–1048. [PMC free article] [PubMed] [Google Scholar]
- Rizzo R. J., McCarthy W. J., Dixit S. N., Lilly M. P., Shively V. P., Flinn W. R., Yao J. S. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989 Oct;10(4):365–373. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Bennett M. R. Death by any other name. Am J Pathol. 1995 Aug;147(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Sumner D. S., Hokanson D. E., Strandness D. E., Jr Stress-strain characteristics and collagen-elastin content of abdominal aortic aneurysms. Surg Gynecol Obstet. 1970 Mar;130(3):459–466. [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B., Ramirez F., Byers P. H. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet. 1989 May;82(2):104–108. doi: 10.1007/BF00284038. [DOI] [PubMed] [Google Scholar]
- Symonds H., Krall L., Remington L., Saenz-Robles M., Lowe S., Jacks T., Van Dyke T. p53-dependent apoptosis suppresses tumor growth and progression in vivo. Cell. 1994 Aug 26;78(4):703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Shah M. R., Pearce W. H., Koch A. E. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994 Oct;42(3-4):159–162. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Komori K., Okadome K., Sugimachi K., Mori R. Detection of active cytomegalovirus infection in inflammatory aortic aneurysms with RNA polymerase chain reaction. J Vasc Surg. 1994 Aug;20(2):235–243. doi: 10.1016/0741-5214(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Thompson R. W. Basic science of abdominal aortic aneurysms: emerging therapeutic strategies for an unresolved clinical problem. Curr Opin Cardiol. 1996 Sep;11(5):504–518. doi: 10.1097/00001573-199609000-00010. [DOI] [PubMed] [Google Scholar]
- Thompson R. W., Holmes D. R., Mertens R. A., Liao S., Botney M. D., Mecham R. P., Welgus H. G., Parks W. C. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995 Jul;96(1):318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson M. D. Histochemistry of aortic elastin in patients with nonspecific abdominal aortic aneurysmal disease. Arch Surg. 1988 Apr;123(4):503–505. doi: 10.1001/archsurg.1988.01400280113023. [DOI] [PubMed] [Google Scholar]
- Tromp G., Wu Y., Prockop D. J., Madhatheri S. L., Kleinert C., Earley J. J., Zhuang J., Norrgård O., Darling R. C., Abbott W. M. Sequencing of cDNA from 50 unrelated patients reveals that mutations in the triple-helical domain of type III procollagen are an infrequent cause of aortic aneurysms. J Clin Invest. 1993 Jun;91(6):2539–2545. doi: 10.1172/JCI116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wang Y., Szekely L., Okan I., Klein G., Wiman K. G. Wild-type p53-triggered apoptosis is inhibited by bcl-2 in a v-myc-induced T-cell lymphoma line. Oncogene. 1993 Dec;8(12):3427–3431. [PubMed] [Google Scholar]
- White J. V., Haas K., Phillips S., Comerota A. J. Adventitial elastolysis is a primary event in aneurysm formation. J Vasc Surg. 1993 Feb;17(2):371–381. doi: 10.1067/mva.1993.43023. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967 Apr;20(4):409–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- Zatina M. A., Zarins C. K., Gewertz B. L., Glagov S. Role of medial lamellar architecture in the pathogenesis of aortic aneurysms. J Vasc Surg. 1984 May;1(3):442–448. [PubMed] [Google Scholar]
- Zhan Q., Carrier F., Fornace A. J., Jr Induction of cellular p53 activity by DNA-damaging agents and growth arrest. Mol Cell Biol. 1993 Jul;13(7):4242–4250. doi: 10.1128/mcb.13.7.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]