Abstract
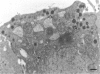
The role of progestins in the pathogenesis of breast cancer in women remains controversial. To advance this discussion, we report the demonstration and localization of progestin-induced biosynthesis of growth hormone (GH) in canine mammary gland tissue. Nontumorous mammary tissues and tumors, both benign and malignant, were obtained from private household dogs. Immunoreactive GH was localized in mammary epithelial cells and correlated with the presence of GH mRNA. Local synthesis of GH was also proven immunoelectron microscopically by demonstrating GH-containing secretory granules. Cellular GH production in nontumorous tissues was more extensive during the progesterone-dominated luteal phase of the ovarian cycle or during exposure to synthetic progestins than during anestrus. GH was also associated with areas of hyperplastic mammary epithelium, which may indicate that locally produced GH enhances proliferation, acting in an autocrine and/or paracrine manner. In 41 of 44 tumors, GH was present. Of 3 GH-negative tumor samples, 2 were from progestin-depleted, castrated bitches. In nonmalignant mammary tissues, GH production is stimulated by progesterone and synthetic progestins interacting with progesterone receptors. In some progesterone-receptor-negative malignant tumors, GH expression was found, indicating loss of this control. Progestin-induced GH probably participates in the cyclic development of the mammary gland but may promote mammary tumorigenesis by stimulating proliferation of susceptible, and sometimes transformed, mammary epithelial cells.
Full text
PDF



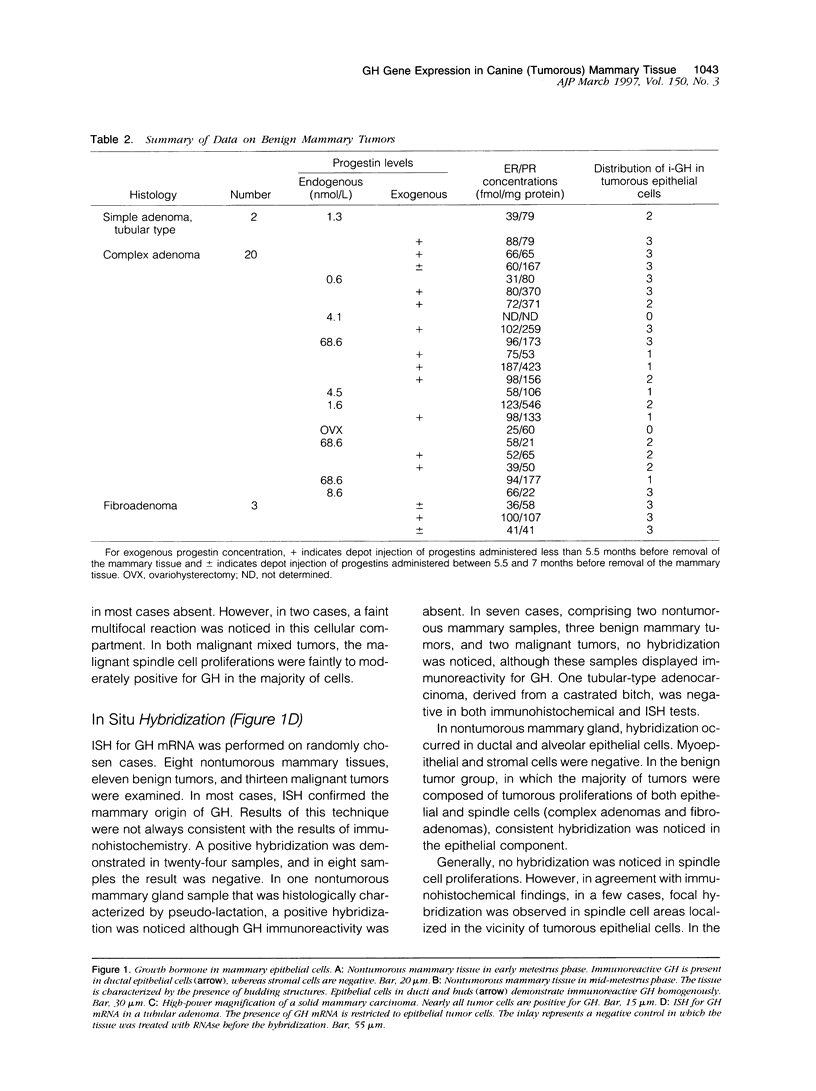
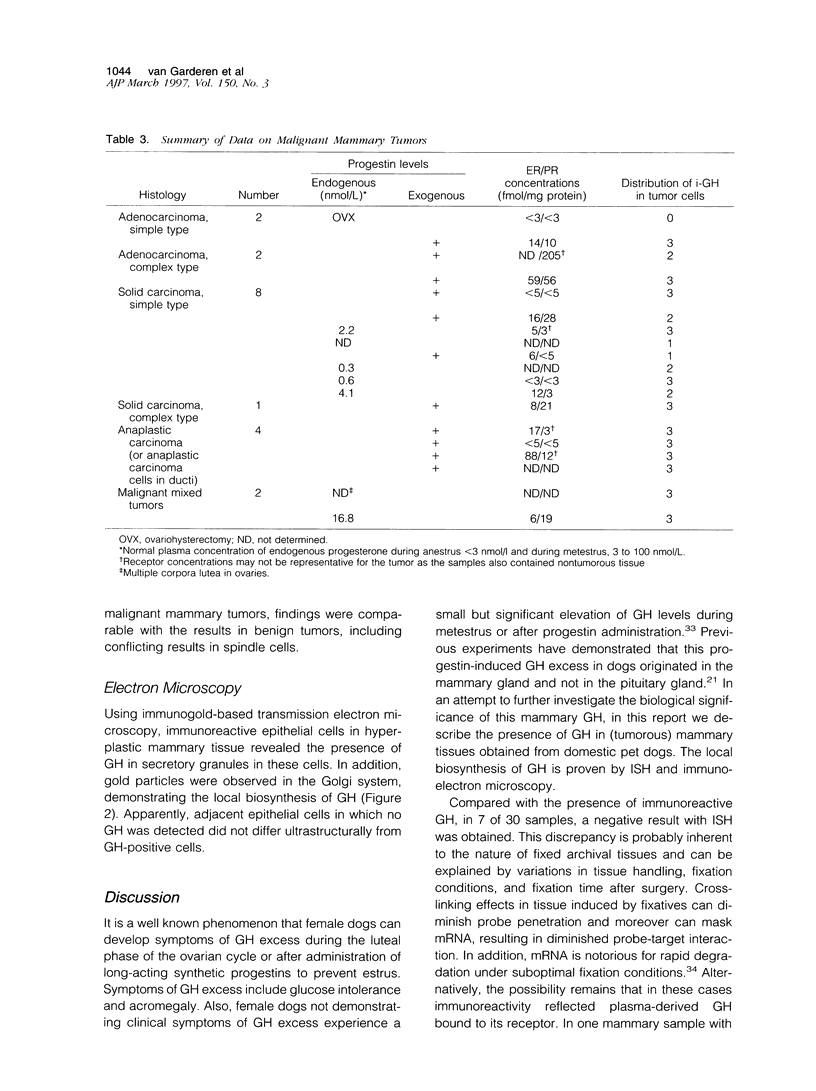



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacus S. S., Zelnick C. R., Plowman G., Yarden Y. Expression of the erbB-2 family of growth factor receptors and their ligands in breast cancers. Implication for tumor biology and clinical behavior. Am J Clin Pathol. 1994 Oct;102(4 Suppl 1):S13–S24. [PubMed] [Google Scholar]
- Casey H. W., Giles R. C., Kwapien R. P. Mammary neoplasia in animals: pathologic aspects and the effects of contraceptive steroids. Recent Results Cancer Res. 1979;66:129–160. doi: 10.1007/978-3-642-81267-5_4. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal K., Bailly A., Rauch C., Quesne M., Milgrom E. Specific binding of progesterone receptor to progesterone-responsive elements does not require prior dimerization. Eur J Biochem. 1993 May 15;214(1):189–195. doi: 10.1111/j.1432-1033.1993.tb17912.x. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Allison A., Martire I., Ellis M., Singer C. Insulin-like growth factor expression in breast cancer epithelium and stroma. Breast Cancer Res Treat. 1992;22(1):21–29. doi: 10.1007/BF01833330. [DOI] [PubMed] [Google Scholar]
- Cullen K. J., Smith H. S., Hill S., Rosen N., Lippman M. E. Growth factor messenger RNA expression by human breast fibroblasts from benign and malignant lesions. Cancer Res. 1991 Sep 15;51(18):4978–4985. [PubMed] [Google Scholar]
- Decouvelaere C., Peyrat J. P., Bonneterre J., Djiane J., Jammes H. Presence of the two growth hormone receptor messenger RNA isoforms in human breast cancer. Cell Growth Differ. 1995 Apr;6(4):477–483. [PubMed] [Google Scholar]
- Ehrlein J., Wanke R., Weis S., Brem G., Hermanns W. Sensitive detection of human growth hormone mRNA in routinely formalin-fixed, paraffin-embedded transgenic mouse tissues by non-isotopic in situ hybridization. Histochemistry. 1994 Aug;102(2):145–152. doi: 10.1007/BF00269018. [DOI] [PubMed] [Google Scholar]
- Fernig D. G., Smith J. A., Rudland P. S. Relationship of growth factors and differentiation in normal and neoplastic development of the mammary gland. Cancer Treat Res. 1991;53:47–78. doi: 10.1007/978-1-4615-3940-7_3. [DOI] [PubMed] [Google Scholar]
- Hampe J. F., Misdorp W. Tumours and dysplasias of the mammary gland. Bull World Health Organ. 1974;50(1-2):111–133. [PMC free article] [PubMed] [Google Scholar]
- Haslam S. Z. Progesterone effects on deoxyribonucleic acid synthesis in normal mouse mammary glands. Endocrinology. 1988 Feb;122(2):464–470. doi: 10.1210/endo-122-2-464. [DOI] [PubMed] [Google Scholar]
- Hayden D. W., Barnes D. M., Johnson K. H. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: a retrospective study. Vet Pathol. 1989 Mar;26(2):104–113. doi: 10.1177/030098588902600202. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr Rev. 1992 May;13(2):146–163. doi: 10.1210/edrv-13-2-146. [DOI] [PubMed] [Google Scholar]
- Jammes H., Gaye P., Belair L., Djiane J. Identification and characterization of growth hormone receptor mRNA in the mammary gland. Mol Cell Endocrinol. 1991 Jan;75(1):27–35. doi: 10.1016/0303-7207(91)90242-k. [DOI] [PubMed] [Google Scholar]
- King R. J. Biology of female sex hormone action in relation to contraceptive agents and neoplasia. Contraception. 1991 Jun;43(6):527–542. doi: 10.1016/0010-7824(91)90002-w. [DOI] [PubMed] [Google Scholar]
- Lemoisson E., Cren H., Goussard J. Chromatographic separation of eight progesterone receptor isoforms in human breast tumors, and detection by radioligand and monoclonal antibodies. Association with hsp90 and hsp70 heat shock proteins. Ann Biol Clin (Paris) 1994;52(6):433–442. [PubMed] [Google Scholar]
- McAndrew J., Fernig D. G., Rudland P. S., Smith J. A. Secretion of transforming growth factor alpha and expression of its receptor in human mammary cell lines. Growth Factors. 1994;10(4):281–287. doi: 10.3109/08977199409010994. [DOI] [PubMed] [Google Scholar]
- Misdorp W. Progestagens and mammary tumours in dogs and cats. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):27–31. [PubMed] [Google Scholar]
- Misdorp W., Romijn A., Hart A. A. Feline mammary tumors: a case-control study of hormonal factors. Anticancer Res. 1991 Sep-Oct;11(5):1793–1797. [PubMed] [Google Scholar]
- Mol J. A., Henzen-Logmans S. C., Hageman P., Misdorp W., Blankenstein M. A., Rijnberk A. Expression of the gene encoding growth hormone in the human mammary gland. J Clin Endocrinol Metab. 1995 Oct;80(10):3094–3096. doi: 10.1210/jcem.80.10.7559904. [DOI] [PubMed] [Google Scholar]
- Mol J. A., van Garderen E., Selman P. J., Wolfswinkel J., Rijinberk A., Rutteman G. R. Growth hormone mRNA in mammary gland tumors of dogs and cats. J Clin Invest. 1995 May;95(5):2028–2034. doi: 10.1172/JCI117888. [DOI] [PMC free article] [PubMed] [Google Scholar]
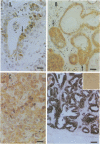
- Nelson L. W., Kelly W. A. Changes in canine mammary gland histology during the estrous cycle. Toxicol Appl Pharmacol. 1974 Jan;27(1):113–122. doi: 10.1016/0041-008x(74)90178-1. [DOI] [PubMed] [Google Scholar]
- Nelson L. W., Kelly W. A. Progestogen-related gross and microscopic changes in female Beagles. Vet Pathol. 1976;13(2):143–156. doi: 10.1177/030098587601300209. [DOI] [PubMed] [Google Scholar]
- Owen L. N., Briggs M. H. Contraceptive steroid toxicology in the Beagle dog and its relevance to human carcinogenicity. Curr Med Res Opin. 1976;4(5):309–329. doi: 10.1185/03007997609109324. [DOI] [PubMed] [Google Scholar]
- Pierce D. F., Jr, Gorska A. E., Chytil A., Meise K. S., Page D. L., Coffey R. J., Jr, Moses H. L. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Henderson B. E., Casagrande J. T., Rosario I., Gray G. E. Oral contraceptive use and early abortion as risk factors for breast cancer in young women. Br J Cancer. 1981 Jan;43(1):72–76. doi: 10.1038/bjc.1981.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Henderson B. E., Krailo M. D., Duke A., Roy S. Breast cancer in young women and use of oral contraceptives: possible modifying effect of formulation and age at use. Lancet. 1983 Oct 22;2(8356):926–930. doi: 10.1016/s0140-6736(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Spicer D. V., Dahmoush L., Press M. F. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein M. S., Barbieri R. L., Friedman A. J. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995 Jan;172(1 Pt 1):14–18. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Rookus M. A., van Leeuwen F. E. Oral contraceptives and risk of breast cancer in women aged 20-54 years. Netherlands Oral Contraceptives and Breast Cancer Study Group. Lancet. 1994 Sep 24;344(8926):844–851. doi: 10.1016/s0140-6736(94)92826-6. [DOI] [PubMed] [Google Scholar]
- Russo J., Gusterson B. A., Rogers A. E., Russo I. H., Wellings S. R., van Zwieten M. J. Comparative study of human and rat mammary tumorigenesis. Lab Invest. 1990 Mar;62(3):244–278. [PubMed] [Google Scholar]
- Rutteman G. R. Contraceptive steroids and the mammary gland: is there a hazard?--Insights from animal studies. Breast Cancer Res Treat. 1992;23(1-2):29–41. doi: 10.1007/BF01831473. [DOI] [PubMed] [Google Scholar]
- Rutteman G. R., Misdorp W., Blankenstein M. A., van den Brom W. E. Oestrogen (ER) and progestin receptors (PR) in mammary tissue of the female dog: different receptor profile in non-malignant and malignant states. Br J Cancer. 1988 Nov;58(5):594–599. doi: 10.1038/bjc.1988.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Dorn C. R., Taylor D. O. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. 1969 Dec;43(6):1249–1261. [PubMed] [Google Scholar]
- Selman P. J., Mol J. A., Rutteman G. R., Rijnberk A. Progestins and growth hormone excess in the dog. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):42–47. [PubMed] [Google Scholar]
- Selman P. J., Mol J. A., Rutteman G. R., van Garderen E., Rijnberk A. Progestin-induced growth hormone excess in the dog originates in the mammary gland. Endocrinology. 1994 Jan;134(1):287–292. doi: 10.1210/endo.134.1.7506206. [DOI] [PubMed] [Google Scholar]
- Shi Y. E., Liu Y. E., Lippman M. E., Dickson R. B. Progestins and antiprogestins in mammary tumour growth and metastasis. Hum Reprod. 1994 Jun;9 (Suppl 1):162–173. doi: 10.1093/humrep/9.suppl_1.162. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Terenghi G., Fallon R. A. Techniques and applications of in situ hybridisation. Curr Top Pathol. 1990;82:289–337. doi: 10.1007/978-3-642-74668-0_7. [DOI] [PubMed] [Google Scholar]
- Wang S., Counterman L. J., Haslam S. Z. Progesterone action in normal mouse mammary gland. Endocrinology. 1990 Nov;127(5):2183–2189. doi: 10.1210/endo-127-5-2183. [DOI] [PubMed] [Google Scholar]
- el-Etreby M. F., Gräf K. J. Effect of contraceptive steroids on mammary gland of beagle dog and its relevance to human carcinogenicity. Pharmacol Ther B. 1979;5(1-3):369–402. doi: 10.1016/0163-7258(79)90107-4. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F. E. Epidemiologic aspects of exogenous progestagens in relation to their role in pathogenesis of human breast cancer. Acta Endocrinol (Copenh) 1991;125 (Suppl 1):13–26. [PubMed] [Google Scholar]