Abstract
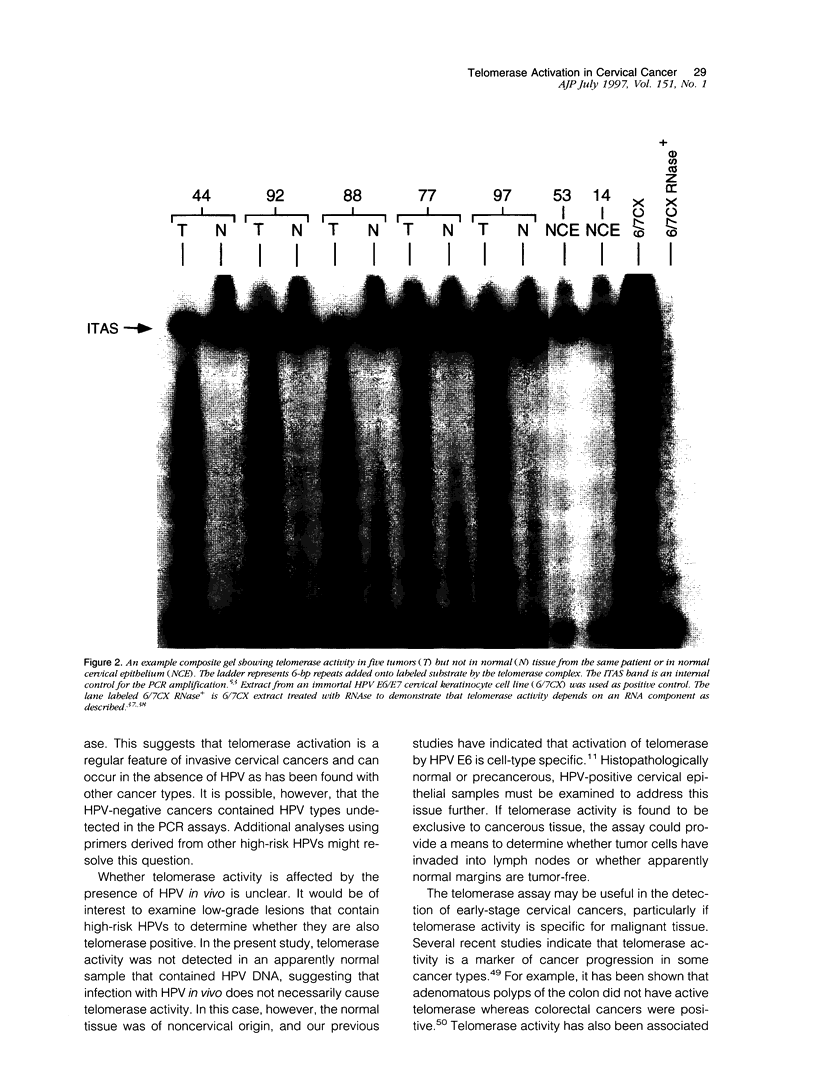
It has been hypothesized that infection with high-risk human papillomaviruses (HPVs), in conjunction with other cellular events, plays a critical role in the development of cervical cancer. Activation of telomerase, a ribonucleoprotein enzyme complex that synthesizes telomere repeats, has been associated with acquisition of the immortal phenotype in vitro and is commonly observed in human cancers. In this study, we have examined 10 high-grade cervical cancers for telomerase activity and for the presence of HPV. Telomerase activity was detected in all of the cancers but in none of the paired histopathologically normal uterine tissues or in normal cervical epithelium. Analysis of these same tissues for HPV nucleic acids by polymerase chain reaction (PCR) using primers from the HPV L1 and E6 open reading frames demonstrated that 7 of 10 cancers were positive for HPV, 3 for HPV type 16 (HPV-16), and 4 for HPV-18. In one case, HPV-16 was detected in histopathologically normal uterine tissue, the same type as that detected in the cancer from the same patient. HPV DNA was not detected in 3 of 10 cancers. These results indicate that telomerase activation is common in high-grade cervical cancers and suggests that telomerase activity may be a useful diagnostic marker for the disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion A. A., Piatyszek M. A., Gupta J., Shay J. W., Bacchetti S., Greider C. W. Human telomerase RNA and telomerase activity in immortal cell lines and tumor tissues. Cancer Res. 1996 Feb 1;56(3):645–650. [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Broccoli D., Young J. W., de Lange T. Telomerase activity in normal and malignant hematopoietic cells. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeneau C., Hay K., Hirte H. W., Gallinger S., Bacchetti S. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 1995 Jun 15;55(12):2533–2536. [PubMed] [Google Scholar]
- Chen J. J., Reid C. E., Band V., Androphy E. J. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science. 1995 Jul 28;269(5223):529–531. doi: 10.1126/science.7624774. [DOI] [PubMed] [Google Scholar]
- Cohn M., Blackburn E. H. Telomerase in yeast. Science. 1995 Jul 21;269(5222):396–400. doi: 10.1126/science.7618104. [DOI] [PubMed] [Google Scholar]
- Collins K., Kobayashi R., Greider C. W. Purification of Tetrahymena telomerase and cloning of genes encoding the two protein components of the enzyme. Cell. 1995 Jun 2;81(5):677–686. doi: 10.1016/0092-8674(95)90529-4. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Botelho F. M., Wang P., Harley C. B., Bacchetti S. Stabilization of short telomeres and telomerase activity accompany immortalization of Epstein-Barr virus-transformed human B lymphocytes. J Virol. 1994 May;68(5):3410–3414. doi: 10.1128/jvi.68.5.3410-3414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., McLachlin C. M. Cervical intraepithelial neoplasia. J Cell Biochem Suppl. 1995;23:71–79. doi: 10.1002/jcb.240590910. [DOI] [PubMed] [Google Scholar]
- Desaintes C., Hallez S., Van Alphen P., Burny A. Transcriptional activation of several heterologous promoters by the E6 protein of human papillomavirus type 16. J Virol. 1992 Jan;66(1):325–333. doi: 10.1128/jvi.66.1.325-333.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Kim N. W., Prowse K. R., Weinrich S. L., Hirsch K. S., West M. D., Bacchetti S., Hirte H. W., Counter C. M., Greider C. W. Telomerase, cell immortality, and cancer. Cold Spring Harb Symp Quant Biol. 1994;59:307–315. doi: 10.1101/sqb.1994.059.01.035. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harrington L., Hull C., Crittenden J., Greider C. Gel shift and UV cross-linking analysis of Tetrahymena telomerase. J Biol Chem. 1995 Apr 14;270(15):8893–8901. doi: 10.1074/jbc.270.15.8893. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Vousden K. H., Hubbert N. L., Lowy D. R., Schiller J. T. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989 Dec 1;8(12):3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama E., Gollahon L., Kataoka T., Kuroi K., Yokoyama T., Gazdar A. F., Hiyama K., Piatyszek M. A., Shay J. W. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996 Jan 17;88(2):116–122. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Hiyama K., Yokoyama T., Matsuura Y., Piatyszek M. A., Shay J. W. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995 Mar;1(3):249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Howley P. M. Role of the human papillomaviruses in human cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5019s–5022s. [PubMed] [Google Scholar]
- Hudson J. B., Bedell M. A., McCance D. J., Laiminis L. A. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990 Feb;64(2):519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., McDougall J. K., Cone R. Immortalization of primary human epithelial cells by cloned cervical carcinoma DNA containing human papillomavirus type 16 E6/E7 open reading frames. J Gen Virol. 1989 May;70(Pt 5):1261–1266. doi: 10.1099/0022-1317-70-5-1261. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Klingelhutz A. J., Barber S. A., Smith P. P., Dyer K., McDougall J. K. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol. 1994 Feb;14(2):961–969. doi: 10.1128/mcb.14.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhutz A. J., Foster S. A., McDougall J. K. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996 Mar 7;380(6569):79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W., Harley C. B. Telomere end-replication problem and cell aging. J Mol Biol. 1992 Jun 20;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Lindsey J., McGill N. I., Lindsey L. A., Green D. K., Cooke H. J. In vivo loss of telomeric repeats with age in humans. Mutat Res. 1991 Jan;256(1):45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Linskens M. H., Harley C. B., West M. D., Campisi J., Hayflick L. Replicative senescence and cell death. Science. 1995 Jan 6;267(5194):17–17. doi: 10.1126/science.7848496. [DOI] [PubMed] [Google Scholar]
- Lungu O., Wright T. C., Jr, Silverstein S. Typing of human papillomaviruses by polymerase chain reaction amplification with L1 consensus primers and RFLP analysis. Mol Cell Probes. 1992 Apr;6(2):145–152. doi: 10.1016/0890-8508(92)90059-7. [DOI] [PubMed] [Google Scholar]
- McDougall J. K. Immortalization and transformation of human cells by human papillomavirus. Curr Top Microbiol Immunol. 1994;186:101–119. doi: 10.1007/978-3-642-78487-3_6. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick R. M., Cornelissen M. T., Wright D. K., Eichinger G. H., Fox H. S., ter Schegget J., Manos M. M. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990 Sep 19;82(18):1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Wright W. E. Telomerase activity in human cancer. Curr Opin Oncol. 1996 Jan;8(1):66–71. doi: 10.1097/00001622-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Thomas M., Massimi P., Jenkins J., Banks L. HPV-18 E6 mediated inhibition of p53 DNA binding activity is independent of E6 induced degradation. Oncogene. 1995 Jan 19;10(2):261–268. [PubMed] [Google Scholar]
- Van der Haegen B. A., Shay J. W. Immortalization of human mammary epithelial cells by SV40 large T-antigen involves a two step mechanism. In Vitro Cell Dev Biol. 1993 Mar;29A(3 Pt 1):180–182. doi: 10.1007/BF02634177. [DOI] [PubMed] [Google Scholar]
- Werness B. A., Levine A. J., Howley P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990 Apr 6;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Piatyszek M. A., Rainey W. E., Byrd W., Shay J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W., Piatyszek M. A. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995 Sep 25;23(18):3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Shay J. W. Telomere positional effects and the regulation of cellular senescence. Trends Genet. 1992 Jun;8(6):193–197. doi: 10.1016/0168-9525(92)90232-s. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]