Abstract
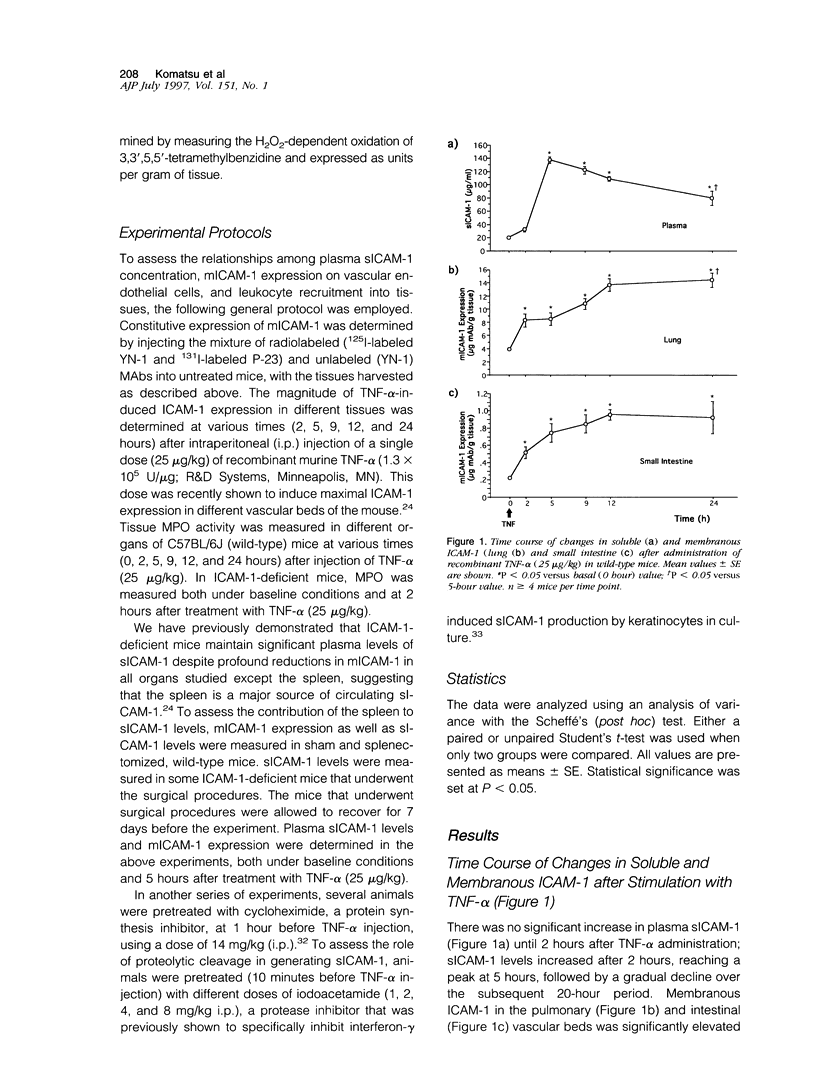
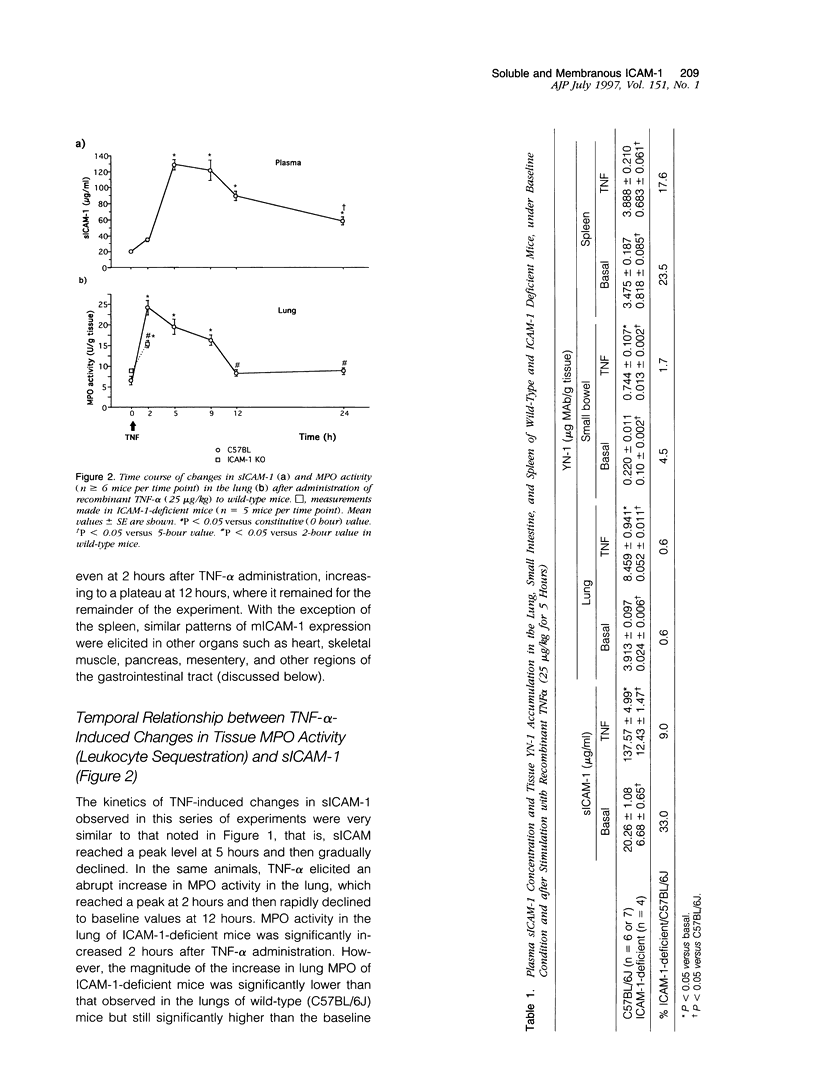
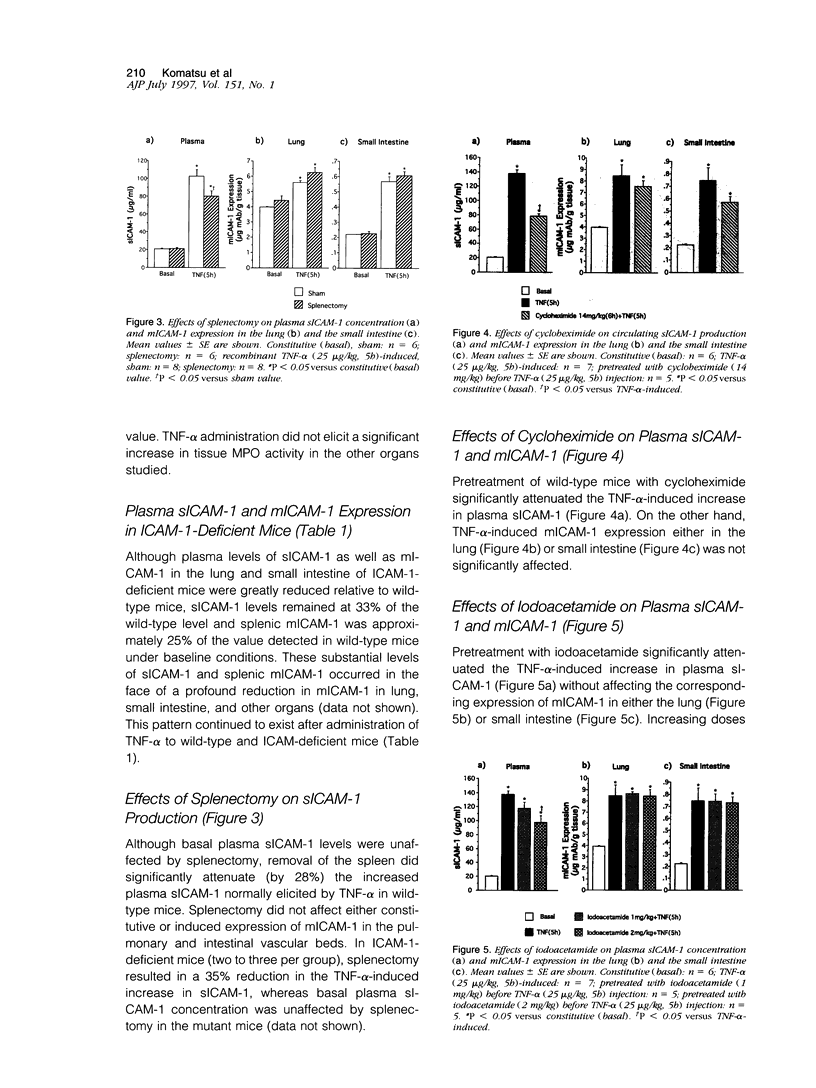
Although circulating levels of soluble intercellular adhesion molecule-1 (sICAM-1) are frequently used as an indicator of the severity of different immune, inflammatory, or neoplastic diseases, little is known about the factors that govern plasma sICAM-1 concentration and its relationship to the membranous form of ICAM-1 (mICAM-1) expressed on vascular endothelial cells. Plasma sICAM-1 concentration (measured by enzyme-linked immunosorbent assay) and mICAM-1 expression (measured using the dual radiolabeled monoclonal antibody technique) in different vascular beds (eg, lung, small intestine, and spleen) were monitored in wild-type (C57BL) and ICAM-1-deficient mice, before and after administration of tumor necrosis factor (TNF)-alpha. In wild-type mice, TNF-alpha elicited time-dependent increases in lung and intestine mICAM-1 (plateau achieved at 12 hours), with a corresponding increase in plasma sICAM-1 (peaked at 5 hours and then declined). The initial increases in mICAM-1 and pulmonary leukocyte sequestration (measured as lung myeloperoxidase activity) induced by TNF-alpha preceded any detectable elevation in sICAM-1. In ICAM-1-deficient mice, plasma sICAM-1 was reduced by approximately 70%, with > 95% reductions of mICAM-1 in lung and intestine, and > 75% reduction in splenic accumulation of anti-ICAM-1 antibody. Although TNF-alpha doubled plasma sICAM-1 in ICAM-1-deficient mice, mICAM-1 was unaffected in all tissues. Either splenectomy or pretreatment with cycloheximide resulted in an attenuated TNF-induced increase in sICAM-1, without affecting mICAM-1 expression. These findings indicate that plasma sICAM-1 concentration does not accurately reflect the level of ICAM-1 expression on endothelial cells in different vascular beds.
Full text
PDF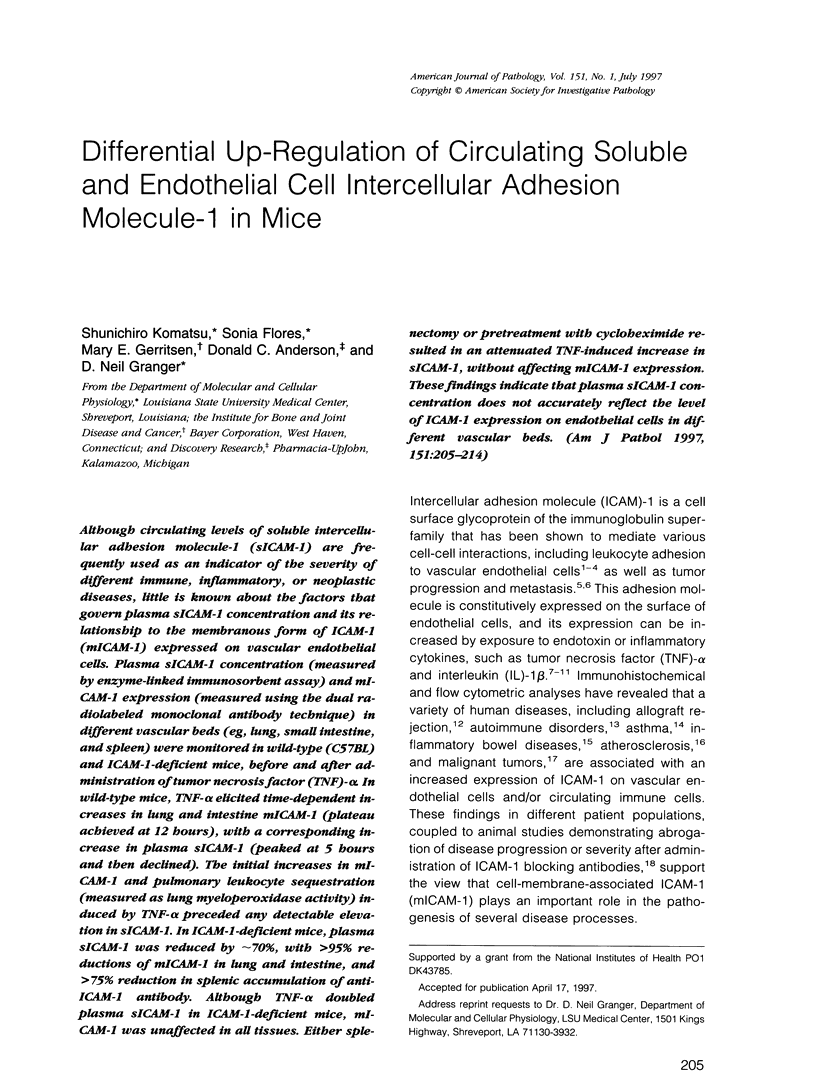






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Budnik A., Grewe M., Gyufko K., Krutmann J. Analysis of the production of soluble ICAM-1 molecules by human cells. Exp Hematol. 1996 Feb;24(2):352–359. [PubMed] [Google Scholar]
- De Rose V., Rolla G., Bucca C., Ghio P., Bertoletti M., Baderna P., Pozzi E. Intercellular adhesion molecule-1 is upregulated on peripheral blood T lymphocyte subsets in dual asthmatic responders. J Clin Invest. 1994 Nov;94(5):1840–1845. doi: 10.1172/JCI117533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchow J., Marchant A., Delville J. P., Schandene L., Goldman M. Upregulation of adhesion molecules induced by broncho-vaxom on phagocytic cells. Int J Immunopharmacol. 1992 Jul;14(5):761–766. doi: 10.1016/0192-0561(92)90073-t. [DOI] [PubMed] [Google Scholar]
- Eppihimer M. J., Wolitzky B., Anderson D. C., Labow M. A., Granger D. N. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996 Sep;79(3):560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Skinner M. P., Berndt M. C., Vadas M. A. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science. 1990 Jul 27;249(4967):414–417. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Newman W. Circulating adhesion molecules in disease. Immunol Today. 1993 Oct;14(10):506–512. doi: 10.1016/0167-5699(93)90267-O. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Specian R. D., Zimmerman T. E. Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J Pharmacol Exp Ther. 1994 Nov;271(2):1114–1121. [PubMed] [Google Scholar]
- Gruschwitz M. S., Hornstein O. P., von Den Driesch P. Correlation of soluble adhesion molecules in the peripheral blood of scleroderma patients with their in situ expression and with disease activity. Arthritis Rheum. 1995 Feb;38(2):184–189. doi: 10.1002/art.1780380206. [DOI] [PubMed] [Google Scholar]
- Haught W. H., Mansour M., Rothlein R., Kishimoto T. K., Mainolfi E. A., Hendricks J. B., Hendricks C., Mehta J. L. Alterations in circulating intercellular adhesion molecule-1 and L-selectin: further evidence for chronic inflammation in ischemic heart disease. Am Heart J. 1996 Jul;132(1 Pt 1):1–8. doi: 10.1016/s0002-8703(96)90383-x. [DOI] [PubMed] [Google Scholar]
- Henninger D. D., Panés J., Eppihimer M., Russell J., Gerritsen M., Anderson D. C., Granger D. N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997 Feb 15;158(4):1825–1832. [PubMed] [Google Scholar]
- Jaeschke H., Essani N. A., Fisher M. A., Vonderfecht S. L., Farhood A., Smith C. W. Release of soluble intercellular adhesion molecule 1 into bile and serum in murine endotoxin shock. Hepatology. 1996 Mar;23(3):530–536. doi: 10.1002/hep.510230318. [DOI] [PubMed] [Google Scholar]
- Jones S. C., Banks R. E., Haidar A., Gearing A. J., Hemingway I. K., Ibbotson S. H., Dixon M. F., Axon A. T. Adhesion molecules in inflammatory bowel disease. Gut. 1995 May;36(5):724–730. doi: 10.1136/gut.36.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King P. D., Sandberg E. T., Selvakumar A., Fang P., Beaudet A. L., Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. J Immunol. 1995 Jun 1;154(11):6080–6093. [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kleinman J. G., Beshensky A., Worcester E. M., Brown D. Expression of osteopontin, a urinary inhibitor of stone mineral crystal growth, in rat kidney. Kidney Int. 1995 Jun;47(6):1585–1596. doi: 10.1038/ki.1995.222. [DOI] [PubMed] [Google Scholar]
- Komatsu S., Panés J., Russell J. M., Anderson D. C., Muzykantov V. R., Miyasaka M., Granger D. N. Effects of chronic arterial hypertension on constitutive and induced intercellular adhesion molecule-1 expression in vivo. Hypertension. 1997 Feb;29(2):683–689. doi: 10.1161/01.hyp.29.2.683. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Anderson D. C., Granger D. N. Role of neutrophil-endothelial cell adhesion in inflammatory disorders. J Crit Care. 1994 Mar;9(1):47–71. doi: 10.1016/0883-9441(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Leeuwenberg J. F., Smeets E. F., Neefjes J. J., Shaffer M. A., Cinek T., Jeunhomme T. M., Ahern T. J., Buurman W. A. E-selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology. 1992 Dec;77(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Ma L., Raycroft L., Asa D., Anderson D. C., Geng J. G. A sialoglycoprotein from human leukocytes functions as a ligand for P-selectin. J Biol Chem. 1994 Nov 4;269(44):27739–27746. [PubMed] [Google Scholar]
- Meyer D. M., Dustin M. L., Carron C. P. Characterization of intercellular adhesion molecule-1 ectodomain (sICAM-1) as an inhibitor of lymphocyte function-associated molecule-1 interaction with ICAM-1. J Immunol. 1995 Oct 1;155(7):3578–3584. [PubMed] [Google Scholar]
- Momosaki S., Yano H., Ogasawara S., Higaki K., Hisaka T., Kojiro M. Expression of intercellular adhesion molecule 1 in human hepatocellular carcinoma. Hepatology. 1995 Dec;22(6):1708–1713. [PubMed] [Google Scholar]
- Myers C. L., Wertheimer S. J., Schembri-King J., Parks T., Wallace R. W. Induction of ICAM-1 by TNF-alpha, IL-1 beta, and LPS in human endothelial cells after downregulation of PKC. Am J Physiol. 1992 Oct;263(4 Pt 1):C767–C772. doi: 10.1152/ajpcell.1992.263.4.C767. [DOI] [PubMed] [Google Scholar]
- Natali P., Nicotra M. R., Cavaliere R., Bigotti A., Romano G., Temponi M., Ferrone S. Differential expression of intercellular adhesion molecule 1 in primary and metastatic melanoma lesions. Cancer Res. 1990 Feb 15;50(4):1271–1278. [PubMed] [Google Scholar]
- O'Brien K. D., McDonald T. O., Chait A., Allen M. D., Alpers C. E. Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation. 1996 Feb 15;93(4):672–682. doi: 10.1161/01.cir.93.4.672. [DOI] [PubMed] [Google Scholar]
- Panés J., Perry M. A., Anderson D. C., Manning A., Leone B., Cepinskas G., Rosenbloom C. L., Miyasaka M., Kvietys P. R., Granger D. N. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995 Dec;269(6 Pt 2):H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- Panés J., Perry M. A., Anderson D. C., Muzykantov V. R., Carden D. L., Miyasaka M., Granger D. N. Portal hypertension enhances endotoxin-induced intercellular adhesion molecule 1 up-regulation in the rat. Gastroenterology. 1996 Mar;110(3):866–874. doi: 10.1053/gast.1996.v110.pm8608897. [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Michel U., Albrecht M., Brück W., Wöckel L., Felgenhauer K. Soluble forms of intercellular adhesion molecule-1 (ICAM-1) block lymphocyte attachment to cerebral endothelial cells. J Neuroimmunol. 1995 Jul;60(1-2):9–15. doi: 10.1016/0165-5728(95)00047-6. [DOI] [PubMed] [Google Scholar]
- Rothlein R., Czajkowski M., O'Neill M. M., Marlin S. D., Mainolfi E., Merluzzi V. J. Induction of intercellular adhesion molecule 1 on primary and continuous cell lines by pro-inflammatory cytokines. Regulation by pharmacologic agents and neutralizing antibodies. J Immunol. 1988 Sep 1;141(5):1665–1669. [PubMed] [Google Scholar]
- Rothlein R., Mainolfi E. A., Czajkowski M., Marlin S. D. A form of circulating ICAM-1 in human serum. J Immunol. 1991 Dec 1;147(11):3788–3793. [PubMed] [Google Scholar]
- Seth R., Raymond F. D., Makgoba M. W. Circulating ICAM-1 isoforms: diagnostic prospects for inflammatory and immune disorders. Lancet. 1991 Jul 13;338(8759):83–84. doi: 10.1016/0140-6736(91)90077-3. [DOI] [PubMed] [Google Scholar]
- Sligh J. E., Jr, Ballantyne C. M., Rich S. S., Hawkins H. K., Smith C. W., Bradley A., Beaudet A. L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Tanio J. W., Basu C. B., Albelda S. M., Eisen H. J. Differential expression of the cell adhesion molecules ICAM-1, VCAM-1, and E-selectin in normal and posttransplantation myocardium. Cell adhesion molecule expression in human cardiac allografts. Circulation. 1994 Apr;89(4):1760–1768. doi: 10.1161/01.cir.89.4.1760. [DOI] [PubMed] [Google Scholar]


