Abstract
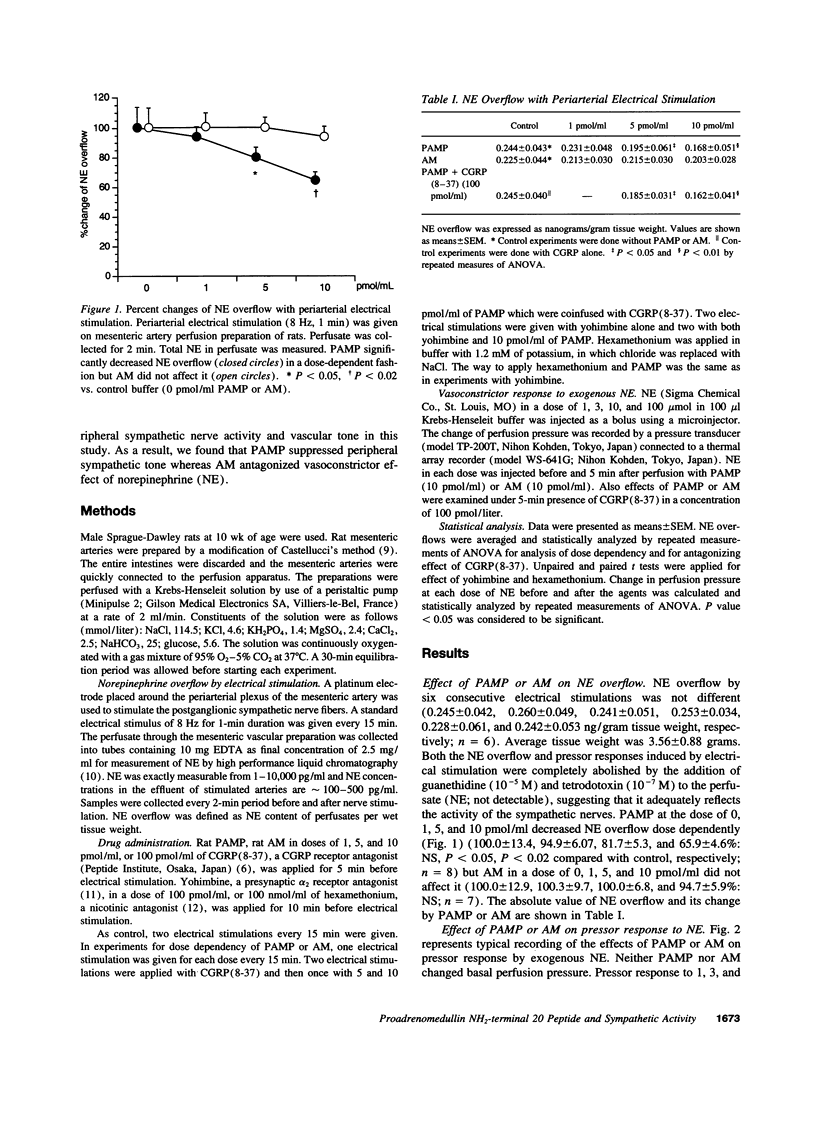
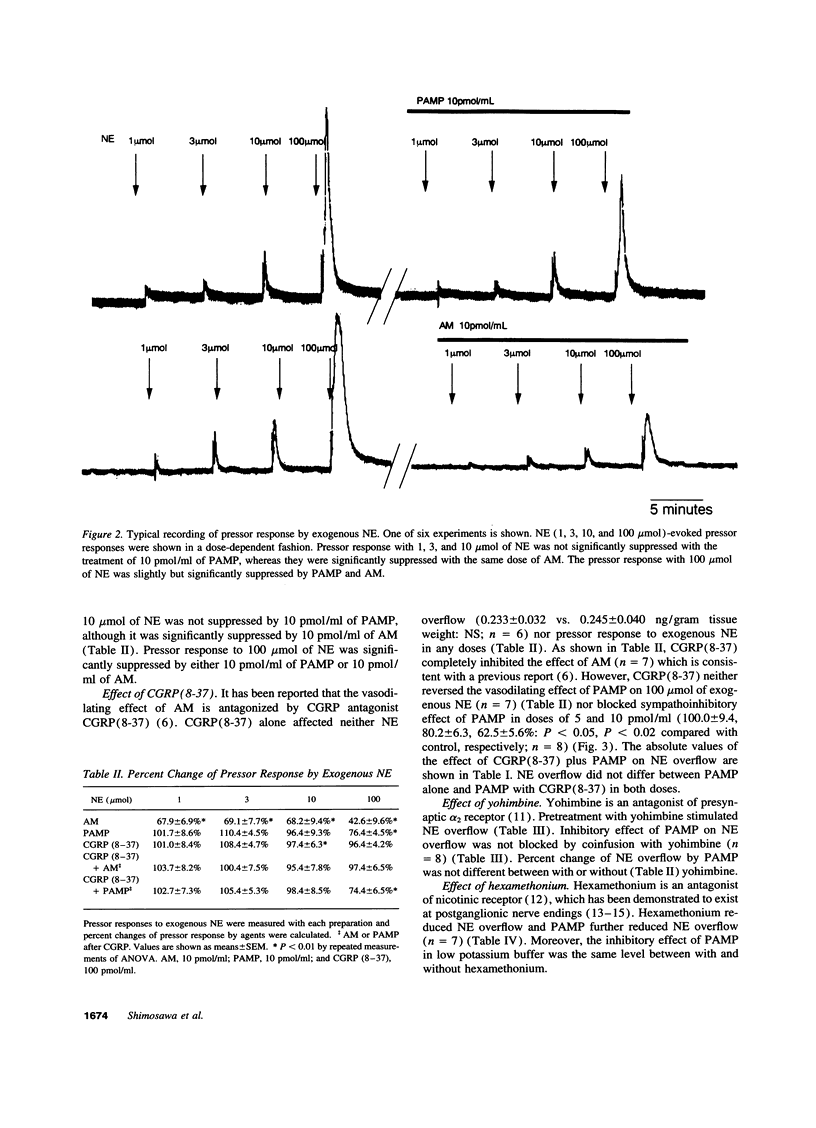
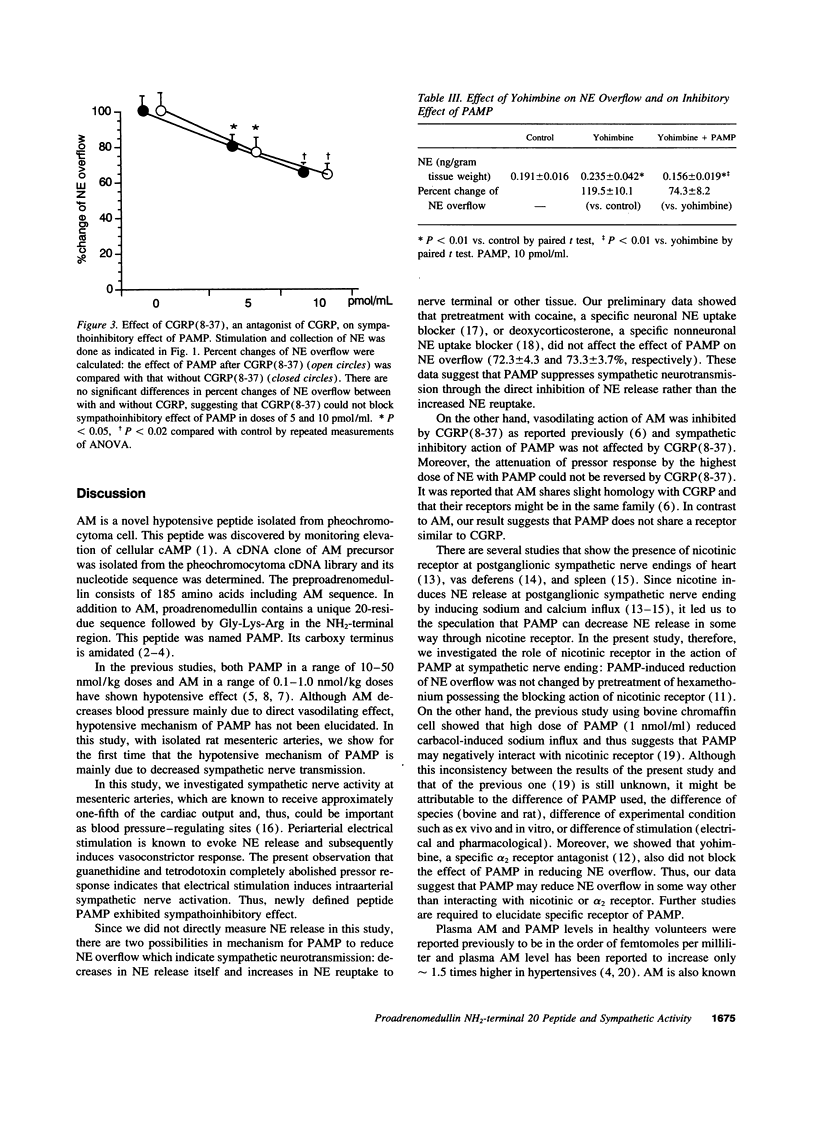
Proadrenomedullin NH(2)-terminal 20 peptide (PAMP) and adrenomedullin, which are derived from proadrenomedullin, exhibit remarkable hypotensive action. We investigated the effect of PAMP and adrenomedullin on peripheral sympathetic neutral transmission. Using perfused rat mesenteric arteries, PAMP (0, 1, 5, and 10 pmol/ml) decreased norepinephrine overflow by periarterial electrical nerve stimulation in a dose-dependent fashion (0.244 +/- 0.043, 0.231 +/- 0.048, 0.195 +/- 0.061 and 0.168 +/- 0.051 ng/gram tissue weigh: NS, P < 0.05, and P < 0.02, respectively). In contrast to PAMP, adrenomedullin (1, 5, and 10 pmol/ml) did not change it. In contrast, vasoconstrictive response of mesenteric arteries to exogenous norepinephrine was significantly attenuated by 10 pmol/ml of adrenomedullin but not by the same dose of PAMP. Calcitonin gene-related peptide (8-37) [CGRP(8-37)], a CGRP receptor antagonist, inhibited the vasodilatory effect of adrenomedullin but could not suppress the sympathoinhibitory effect of PAMP. Neither a nicotinic antagonist, hexamethonium, nor a presynaptic alfa2 antagonist, yohimbine, blocked the sympathoinhibitory effect of PAMP. Thus, it suggests that PAMP and adrenomedullin, which are derived from the same gene, exhibit different hypotensive mechanisms: PAMP inhibits neural transmission at peripheral sympathetic nerve ending, although adrenomedullin directly dilates vascular smooth muscle, possibly through CGRP-like receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ishimitsu T., Nishikimi T., Saito Y., Kitamura K., Eto T., Kangawa K., Matsuo H., Omae T., Matsuoka H. Plasma levels of adrenomedullin, a newly identified hypotensive peptide, in patients with hypertension and renal failure. J Clin Invest. 1994 Nov;94(5):2158–2161. doi: 10.1172/JCI117573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama Y., Kitamura K., Ichiki Y., Nakamura S., Kida O., Kangawa K., Eto T. Hemodynamic effects of a novel hypotensive peptide, human adrenomedullin, in rats. Eur J Pharmacol. 1993 Sep 14;241(2-3):271–273. doi: 10.1016/0014-2999(93)90214-3. [DOI] [PubMed] [Google Scholar]
- Ito Y., Noda H., Isaka M., Ando K., Sato Y., Fujita T. Norepinephrine responsiveness in patients with borderline hypertension under three different sodium balances. Clin Exp Hypertens A. 1989;11 (Suppl 1):363–370. doi: 10.3109/10641968909045442. [DOI] [PubMed] [Google Scholar]
- Jayasundar S., Vohra M. M. Mechanism of nicotine-induced release of noradrenaline from adrenergic nerve endings. Br J Pharmacol. 1977 Oct;61(2):183–188. doi: 10.1111/j.1476-5381.1977.tb08403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh F., Kitamura K., Niina H., Yamamoto R., Washimine H., Kangawa K., Yamamoto Y., Kobayashi H., Eto T., Wada A. Proadrenomedullin N-terminal 20 peptide (PAMP), an endogenous anticholinergic peptide: its exocytotic secretion and inhibition of catecholamine secretion in adrenal medulla. J Neurochem. 1995 Jan;64(1):459–461. doi: 10.1046/j.1471-4159.1995.64010459.x. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Garcia A. G., Prat J. C. Action of nicotine on sympathetic nerve terminals. J Pharmacol Exp Ther. 1980 Apr;213(1):133–138. [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Ishiyama Y., Washimine H., Ichiki Y., Kawamoto M., Minamino N., Matsuo H., Eto T. Identification and hypotensive activity of proadrenomedullin N-terminal 20 peptide (PAMP). FEBS Lett. 1994 Aug 29;351(1):35–37. doi: 10.1016/0014-5793(94)00810-8. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993 Apr 30;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Sakata J., Kangawa K., Kojima M., Matsuo H., Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993 Jul 30;194(2):720–725. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- Löffelholz K. Autoinhibition of nicotinic release of noradrenaline from postganglionic sympathetic nerves. Naunyn Schmiedebergs Arch Pharmakol. 1970;267(1):49–63. doi: 10.1007/BF00997114. [DOI] [PubMed] [Google Scholar]
- Nedergaard O. A., Schrold J. The mechanism of action of nicotine on vascular adrenergic neuroeffector transmission. Eur J Pharmacol. 1977 Apr 21;42(4):315–329. doi: 10.1016/0014-2999(77)90165-0. [DOI] [PubMed] [Google Scholar]
- Nichols A. J., Wilson A. C., Hiley C. R. Effects of chemical sympathectomy with 6-hydroxydopamine on cardiac output and its distribution in the rat. Eur J Pharmacol. 1985 Feb 26;109(2):263–268. doi: 10.1016/0014-2999(85)90428-5. [DOI] [PubMed] [Google Scholar]
- Nishikimi T., Kitamura K., Saito Y., Shimada K., Ishimitsu T., Takamiya M., Kangawa K., Matsuo H., Eto T., Omae T. Clinical studies on the sites of production and clearance of circulating adrenomedullin in human subjects. Hypertension. 1994 Nov;24(5):600–604. doi: 10.1161/01.hyp.24.5.600. [DOI] [PubMed] [Google Scholar]
- Nuki C., Kawasaki H., Kitamura K., Takenaga M., Kangawa K., Eto T., Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem Biophys Res Commun. 1993 Oct 15;196(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- Pitts D. K., Marwah J. Autonomic actions of cocaine. Can J Physiol Pharmacol. 1989 Sep;67(9):1168–1176. doi: 10.1139/y89-186. [DOI] [PubMed] [Google Scholar]
- Sakata J., Shimokubo T., Kitamura K., Nakamura S., Kangawa K., Matsuo H., Eto T. Molecular cloning and biological activities of rat adrenomedullin, a hypotensive peptide. Biochem Biophys Res Commun. 1993 Sep 15;195(2):921–927. doi: 10.1006/bbrc.1993.2132. [DOI] [PubMed] [Google Scholar]
- Salt P. J. Inhibition of noradrenaline uptake 2 in the isolated rat heart by steroids, clonidine and methoxylated phenylethylamines. Eur J Pharmacol. 1972 Dec;20(3):329–340. doi: 10.1016/0014-2999(72)90194-x. [DOI] [PubMed] [Google Scholar]
- Shimosawa T., Ando K., Ono A., Takahashi K., Isshiki M., Kanda M., Ogata E., Fujita T. Insulin inhibits norepinephrine overflow from peripheral sympathetic nerve ending. Biochem Biophys Res Commun. 1992 Oct 15;188(1):330–335. doi: 10.1016/0006-291x(92)92389-f. [DOI] [PubMed] [Google Scholar]
- Sugo S., Minamino N., Kangawa K., Miyamoto K., Kitamura K., Sakata J., Eto T., Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994 Jun 30;201(3):1160–1166. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- Sugo S., Minamino N., Shoji H., Kangawa K., Kitamura K., Eto T., Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1994 Aug 30;203(1):719–726. doi: 10.1006/bbrc.1994.2241. [DOI] [PubMed] [Google Scholar]
- Washimine H., Kitamura K., Ichiki Y., Yamamoto Y., Kangawa K., Matsuo H., Eto T. Immunoreactive proadrenomedullin N-terminal 20 peptide in human tissue, plasma and urine. Biochem Biophys Res Commun. 1994 Jul 29;202(2):1081–1087. doi: 10.1006/bbrc.1994.2039. [DOI] [PubMed] [Google Scholar]


