Abstract
Systemically administered interleukin (IL)-12 causes liver inflammation in mice characterized by Kupffer cell proliferation and hypertrophy, hepatocyte necrosis, and multifocal accumulations of leukocytes in the hepatic parenchyma and around portal tracts and central veins. We have used both immunohistochemical staining and radiolabeled antibody quantitation to examine adhesion molecule expression in the livers of mice dosed daily with murine IL-12. Cells infiltrating livers of IL-12-treated mice were primarily mononuclear leukocytes expressing LFA-1, VLA-4, MAC-1, and CD18 adhesion molecules but little L-selectin. Kupffer cells constitutively expressed LFA-1 and smaller amounts of MAC-1, and high levels of ICAM-1 were constitutively expressed by liver sinusoidal lining cells, portal tract, and central vein endothelia. With IL-12 treatment, existing ICAM-1 expression was up-regulated and de novo expression occurred along bile duct epithelia. VCAM-1 levels were dramatically increased, with induced expression occurring along portal tract and central vein endothelia and scattered bile duct epithelial cells and in aggregations of cells in perivascular areas and the liver parenchyma. Although constitutive expression of E- and P-selectin was negligible, Il-12 induced a moderate rise in E-selectin levels. These increases in adhesion molecule expression may have implications for the therapeutic use of IL-12, especially in patients with liver disease or autoimmune conditions where augmented adhesion molecule expression may be critical to disease pathogenesis.
Full text
PDF


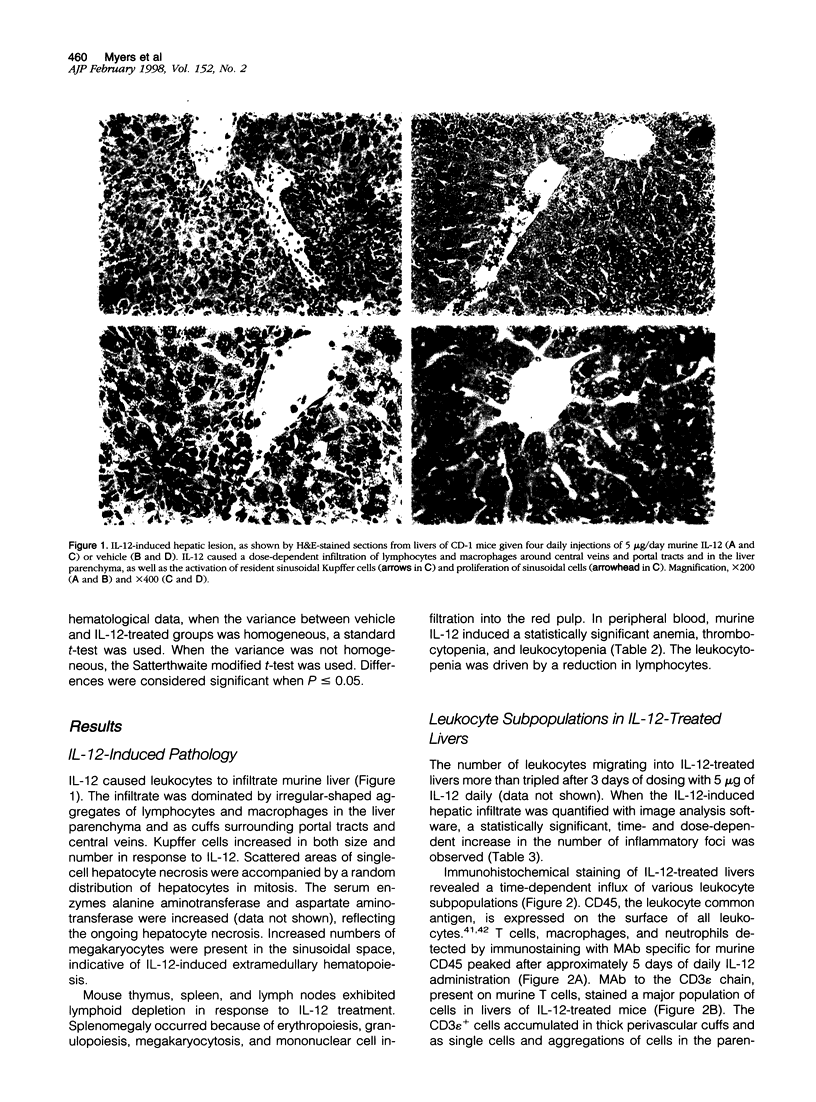
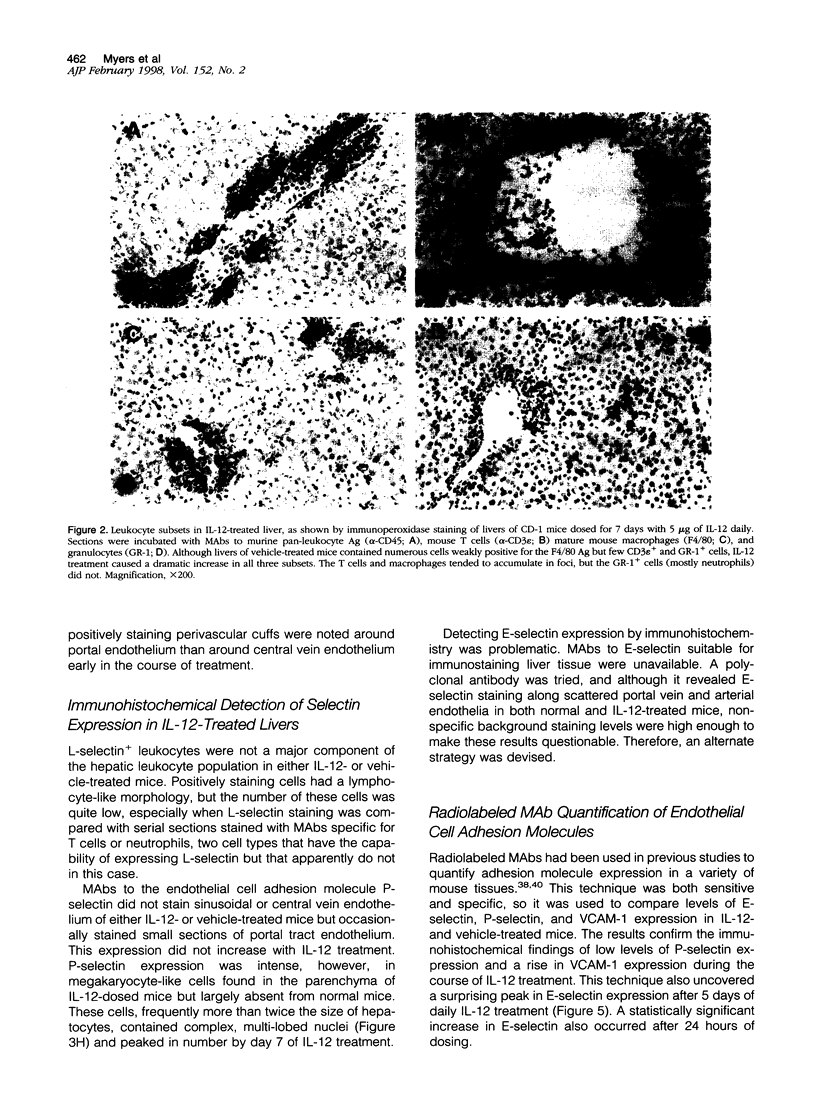
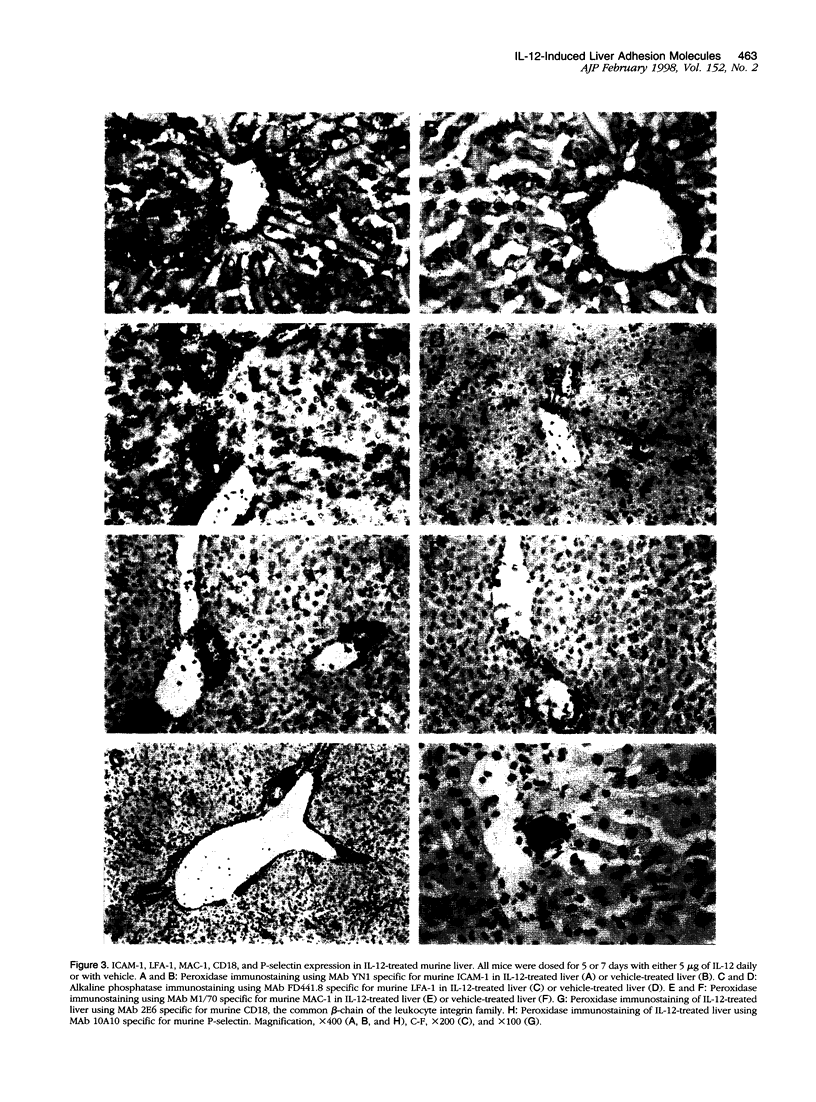
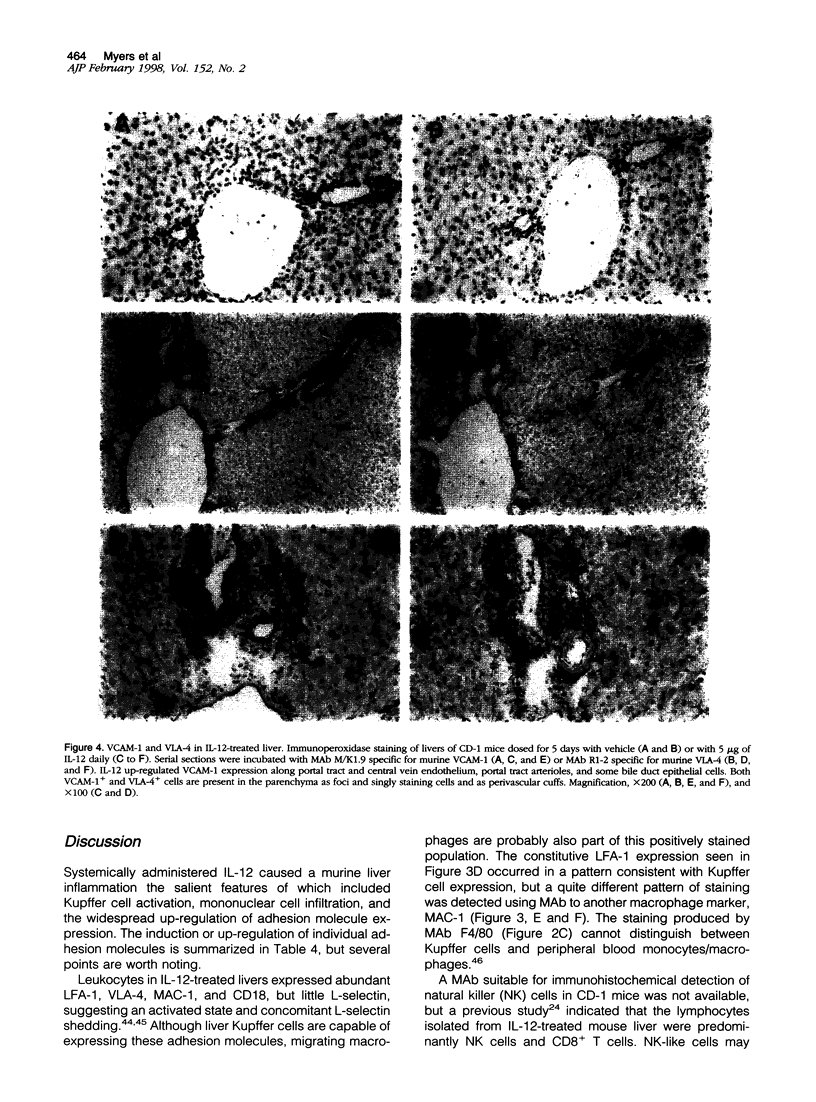
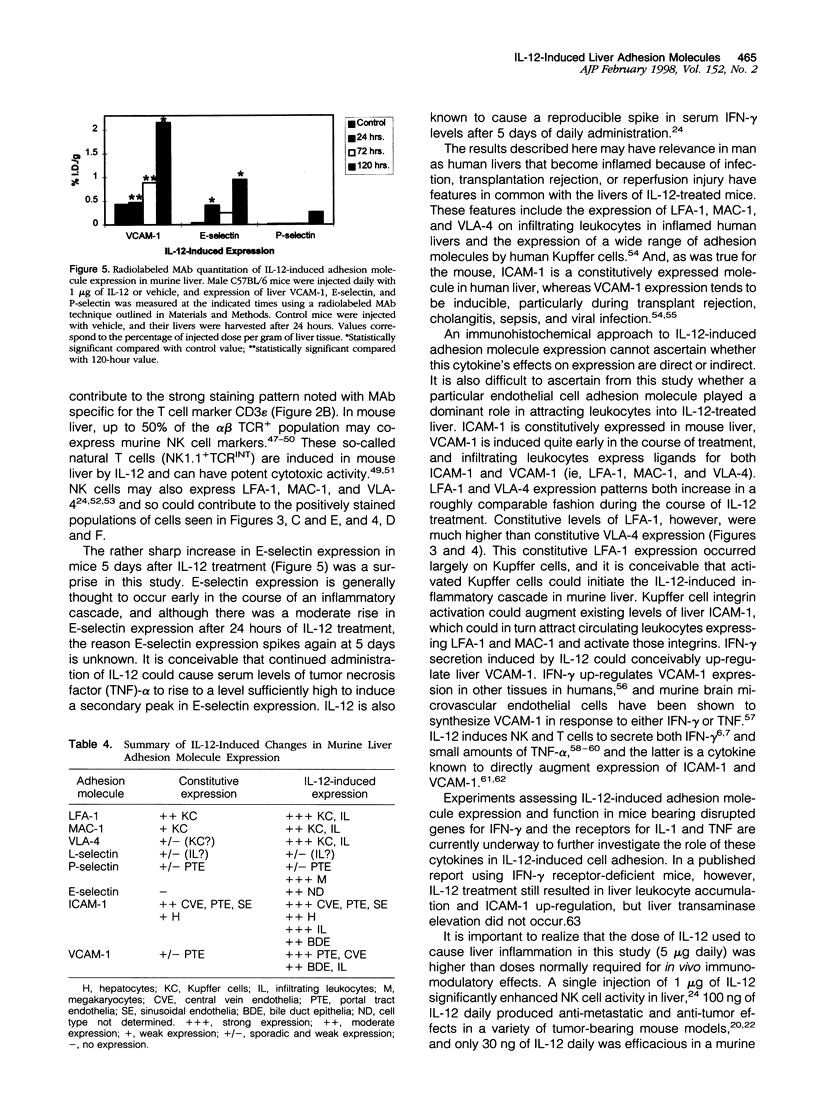



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Smith C. W., Ward P. A. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–512. [PubMed] [Google Scholar]
- Bereta M., Bereta J., Georgoff I., Coffman F. D., Cohen S., Cohen M. C. Methylxanthines and calcium-mobilizing agents inhibit the expression of cytokine-inducible nitric oxide synthase and vascular cell adhesion molecule-1 in murine microvascular endothelial cells. Exp Cell Res. 1994 Jun;212(2):230–242. doi: 10.1006/excr.1994.1139. [DOI] [PubMed] [Google Scholar]
- Bi Z., Quandt P., Komatsu T., Barna M., Reiss C. S. IL-12 promotes enhanced recovery from vesicular stomatitis virus infection of the central nervous system. J Immunol. 1995 Dec 15;155(12):5684–5689. [PubMed] [Google Scholar]
- Bianchi G., Sironi M., Ghibaudi E., Selvaggini C., Elices M., Allavena P., Mantovani A. Migration of natural killer cells across endothelial cell monolayers. J Immunol. 1993 Nov 15;151(10):5135–5144. [PubMed] [Google Scholar]
- Brunda M. J., Luistro L., Warrier R. R., Wright R. B., Hubbard B. R., Murphy M., Wolf S. F., Gately M. K. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993 Oct 1;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Car B. D., Eng V. M., Schnyder B., LeHir M., Shakhov A. N., Woerly G., Huang S., Aguet M., Anderson T. D., Ryffel B. Role of interferon-gamma in interleukin 12-induced pathology in mice. Am J Pathol. 1995 Dec;147(6):1693–1707. [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Leukocyte-endothelial adhesion molecules. Blood. 1994 Oct 1;84(7):2068–2101. [PubMed] [Google Scholar]
- Chan S. H., Perussia B., Gupta J. W., Kobayashi M., Pospísil M., Young H. A., Wolf S. F., Young D., Clark S. C., Trinchieri G. Induction of interferon gamma production by natural killer cell stimulatory factor: characterization of the responder cells and synergy with other inducers. J Exp Med. 1991 Apr 1;173(4):869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons K. V., Brummer E., Stevens D. A. Cytokine treatment of central nervous system infection: efficacy of interleukin-12 alone and synergy with conventional antifungal therapy in experimental cryptococcosis. Antimicrob Agents Chemother. 1994 Mar;38(3):460–464. doi: 10.1128/aac.38.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994 Jun 1;4(6):506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Eppihimer M. J., Wolitzky B., Anderson D. C., Labow M. A., Granger D. N. Heterogeneity of expression of E- and P-selectins in vivo. Circ Res. 1996 Sep;79(3):560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- Flynn J. L., Goldstein M. M., Triebold K. J., Sypek J., Wolf S., Bloom B. R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995 Sep 1;155(5):2515–2524. [PubMed] [Google Scholar]
- Gately M. K., Desai B. B., Wolitzky A. G., Quinn P. M., Dwyer C. M., Podlaski F. J., Familletti P. C., Sinigaglia F., Chizonnite R., Gubler U. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991 Aug 1;147(3):874–882. [PubMed] [Google Scholar]
- Gately M. K., Gubler U., Brunda M. J., Nadeau R. R., Anderson T. D., Lipman J. M., Sarmiento U. Interleukin-12: a cytokine with therapeutic potential in oncology and infectious diseases. Ther Immunol. 1994 Jun;1(3):187–196. [PubMed] [Google Scholar]
- Gately M. K., Warrier R. R., Honasoge S., Carvajal D. M., Faherty D. A., Connaughton S. E., Anderson T. D., Sarmiento U., Hubbard B. R., Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994 Jan;6(1):157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- Gately M. K., Wolitzky A. G., Quinn P. M., Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 1992 Aug;143(1):127–142. doi: 10.1016/0008-8749(92)90011-d. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hieny S., Wynn T. A., Wolf S., Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R. W., Ross E. L., Barker J. N., MacDonald D. M. Vascular cell adhesion molecule-1: expression in normal and diseased skin and regulation in vivo by interferon gamma. J Am Acad Dermatol. 1993 Jul;29(1):67–72. doi: 10.1016/0190-9622(93)70154-l. [DOI] [PubMed] [Google Scholar]
- Hashimoto W., Takeda K., Anzai R., Ogasawara K., Sakihara H., Sugiura K., Seki S., Kumagai K. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995 May 1;154(9):4333–4340. [PubMed] [Google Scholar]
- Heinzel F. P., Schoenhaut D. S., Rerko R. M., Rosser L. E., Gately M. K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993 May 1;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
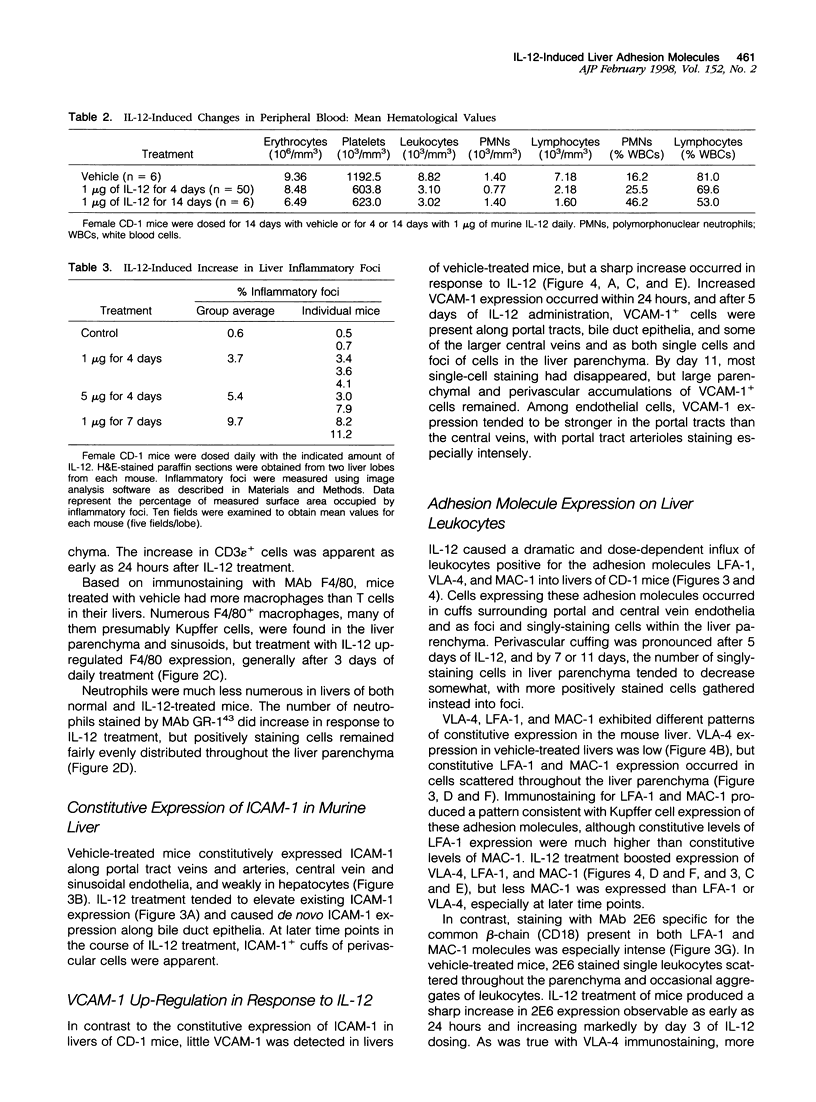
- Henninger D. D., Panés J., Eppihimer M., Russell J., Gerritsen M., Anderson D. C., Granger D. N. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997 Feb 15;158(4):1825–1832. [PubMed] [Google Scholar]
- Hestdal K., Ruscetti F. W., Ihle J. N., Jacobsen S. E., Dubois C. M., Kopp W. C., Longo D. L., Keller J. R. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991 Jul 1;147(1):22–28. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Jung T. M., Gallatin W. M., Weissman I. L., Dailey M. O. Down-regulation of homing receptors after T cell activation. J Immunol. 1988 Dec 15;141(12):4110–4117. [PubMed] [Google Scholar]
- Khan I. A., Matsuura T., Kasper L. H. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994 May;62(5):1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989 Sep 1;170(3):827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lee S. H., Starkey P. M., Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985 Mar 1;161(3):475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Waldburger K. E., Goldman S. J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995 Jan 1;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K. Molecular mechanisms of leucocyte rolling and adhesion to microvascular endothelium. Eur Heart J. 1993 Nov;14 (Suppl 1):68–73. [PubMed] [Google Scholar]
- Manetti R., Parronchi P., Giudizi M. G., Piccinni M. P., Maggi E., Trinchieri G., Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993 Apr 1;177(4):1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpä A., Jäskeläinen J., Carpén O., Patarroyo M., Timonen T. Expression of integrins and other adhesion molecules on NK cells; impact of IL-2 on short- and long-term cultures. Int J Cancer. 1993 Mar 12;53(5):850–855. doi: 10.1002/ijc.2910530524. [DOI] [PubMed] [Google Scholar]
- Nastala C. L., Edington H. D., McKinney T. G., Tahara H., Nalesnik M. A., Brunda M. J., Gately M. K., Wolf S. F., Schreiber R. D., Storkus W. J. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994 Aug 15;153(4):1697–1706. [PubMed] [Google Scholar]
- Naume B., Gately M., Espevik T. A comparative study of IL-12 (cytotoxic lymphocyte maturation factor)-, IL-2-, and IL-7-induced effects on immunomagnetically purified CD56+ NK cells. J Immunol. 1992 Apr 15;148(8):2429–2436. [PubMed] [Google Scholar]
- Neta R., Stiefel S. M., Finkelman F., Herrmann S., Ali N. IL-12 protects bone marrow from and sensitizes intestinal tract to ionizing radiation. J Immunol. 1994 Nov 1;153(9):4230–4237. [PubMed] [Google Scholar]
- Neumann B., Machleidt T., Lifka A., Pfeffer K., Vestweber D., Mak T. W., Holzmann B., Krönke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996 Feb 15;156(4):1587–1593. [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T., MacDonald H. R. Major histocompatibility complex class I related molecules control the development of CD4+8- and CD4-8- subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994 Aug 1;180(2):699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J. S., Wang B., Terhorst C., Biron C. A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995 Oct 1;182(4):1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J. S., Wolf S. F., Biron C. A. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994 Feb 1;152(3):1253–1264. [PubMed] [Google Scholar]
- Panés J., Perry M. A., Anderson D. C., Manning A., Leone B., Cepinskas G., Rosenbloom C. L., Miyasaka M., Kvietys P. R., Granger D. N. Regional differences in constitutive and induced ICAM-1 expression in vivo. Am J Physiol. 1995 Dec;269(6 Pt 2):H1955–H1964. doi: 10.1152/ajpheart.1995.269.6.H1955. [DOI] [PubMed] [Google Scholar]
- Perussia B., Chan S. H., D'Andrea A., Tsuji K., Santoli D., Pospisil M., Young D., Wolf S. F., Trinchieri G. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on the proliferation of TCR-alpha beta+, TCR-gamma delta+ T lymphocytes, and NK cells. J Immunol. 1992 Dec 1;149(11):3495–3502. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Soiffer R. J., Wolf S. F., Manley T. J., Donahue C., Young D., Herrmann S. H., Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992 Mar 1;175(3):779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Löhler J., Schorle H., Klebb G., Haber H., Sickel E., Noelle R. J., Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995 Nov;25(11):3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- Sarmiento U. M., Riley J. H., Knaack P. A., Lipman J. M., Becker J. M., Gately M. K., Chizzonite R., Anderson T. D. Biologic effects of recombinant human interleukin-12 in squirrel monkeys (Sciureus saimiri). Lab Invest. 1994 Dec;71(6):862–873. [PubMed] [Google Scholar]
- Schijns V. E., Haagmans B. L., Horzinek M. C. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-gamma-receptor-deficient mice. J Immunol. 1995 Sep 1;155(5):2525–2532. [PubMed] [Google Scholar]
- Sedegah M., Finkelman F., Hoffman S. L. Interleukin 12 induction of interferon gamma-dependent protection against malaria. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10700–10702. doi: 10.1073/pnas.91.22.10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Abo T., Ohteki T., Sugiura K., Kumagai K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J Immunol. 1991 Aug 15;147(4):1214–1221. [PubMed] [Google Scholar]
- Smith J. W., 2nd, Urba W. J., Curti B. D., Elwood L. J., Steis R. G., Janik J. E., Sharfman W. H., Miller L. L., Fenton R. G., Conlon K. C. The toxic and hematologic effects of interleukin-1 alpha administered in a phase I trial to patients with advanced malignancies. J Clin Oncol. 1992 Jul;10(7):1141–1152. doi: 10.1200/JCO.1992.10.7.1141. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Steinhoff G., Behrend M., Schrader B., Duijvestijn A. M., Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3. Am J Pathol. 1993 Feb;142(2):481–488. [PMC free article] [PubMed] [Google Scholar]
- Steinhoff G., Behrend M., Schrader B., Pichlmayr R. Intercellular immune adhesion molecules in human liver transplants: overview on expression patterns of leukocyte receptor and ligand molecules. Hepatology. 1993 Aug;18(2):440–453. [PubMed] [Google Scholar]
- Sypek J. P., Chung C. L., Mayor S. E., Subramanyam J. M., Goldman S. J., Sieburth D. S., Wolf S. F., Schaub R. G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993 Jun 1;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Ogasawara K., Takeda K., Hashimoto W., Sakihara H., Kumagai K., Anzai R., Satoh M., Seki S. LPS induces NK1.1+ alpha beta T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996 Apr 1;156(7):2436–2442. [PubMed] [Google Scholar]
- Trembleau S., Penna G., Bosi E., Mortara A., Gately M. K., Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995 Feb 1;181(2):817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp C. S., Gately M. K., Hakimi J., Ling P., Unanue E. R. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994 Feb 15;152(4):1883–1887. [PubMed] [Google Scholar]
- Watanabe H., Miyaji C., Kawachi Y., Iiai T., Ohtsuka K., Iwanage T., Takahashi-Iwanaga H., Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995 Sep 15;155(6):2972–2983. [PubMed] [Google Scholar]
- Williamson E., Garside P., Bradley J. A., Mowat A. M. IL-12 is a central mediator of acute graft-versus-host disease in mice. J Immunol. 1996 Jul 15;157(2):689–699. [PubMed] [Google Scholar]
- Wynn T. A., Eltoum I., Oswald I. P., Cheever A. W., Sher A. Endogenous interleukin 12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoni and exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994 May 1;179(5):1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]