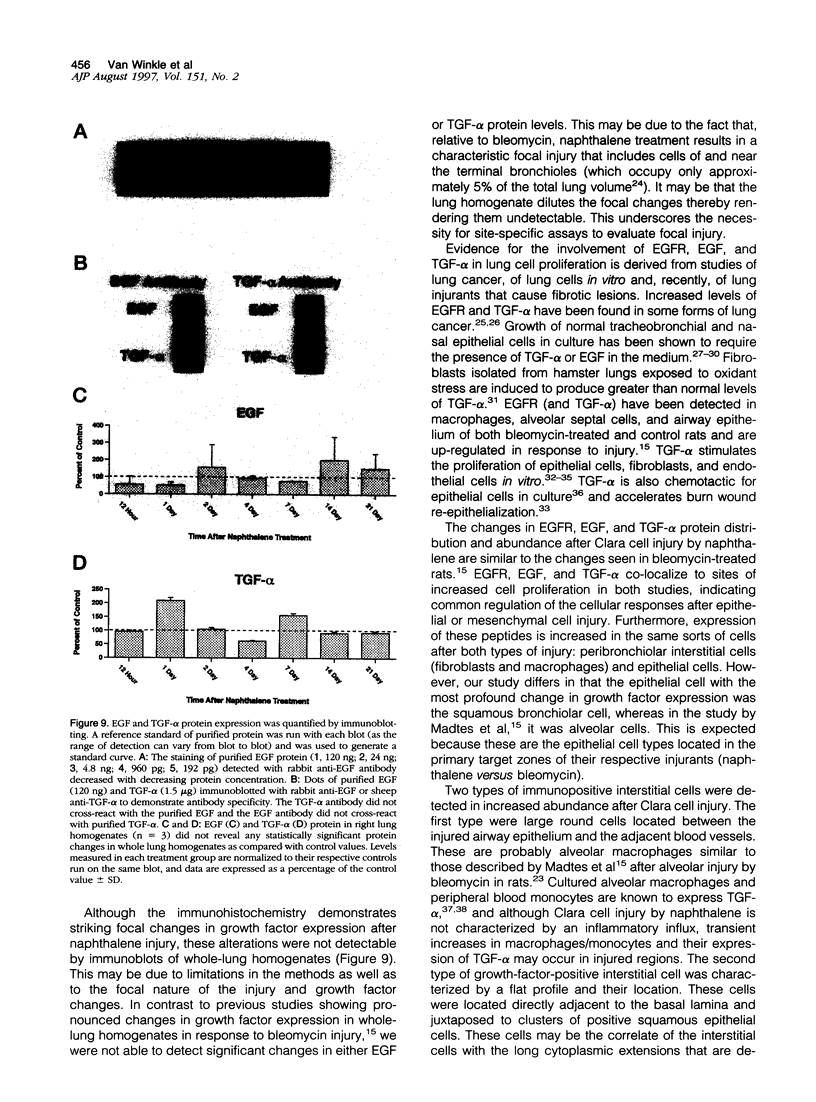
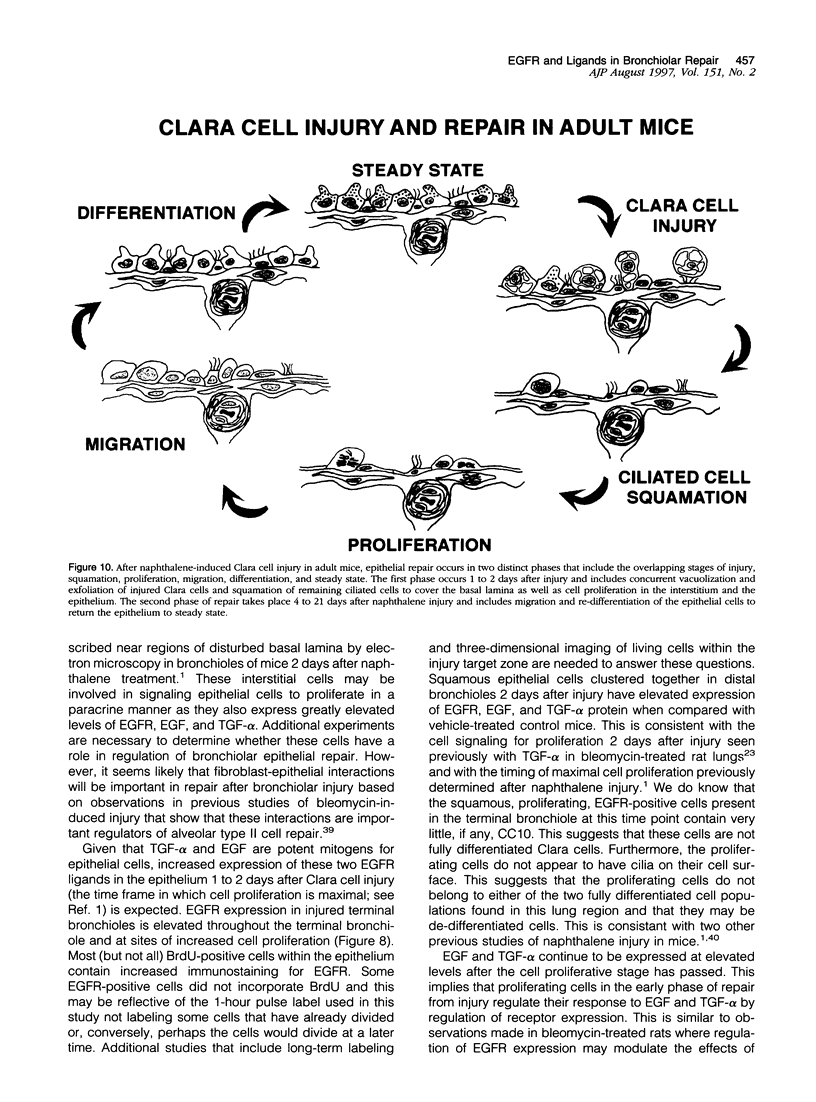
Abstract
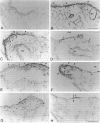
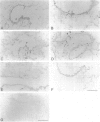
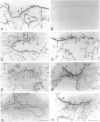
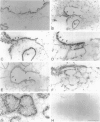
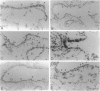
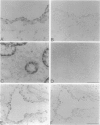
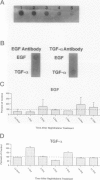
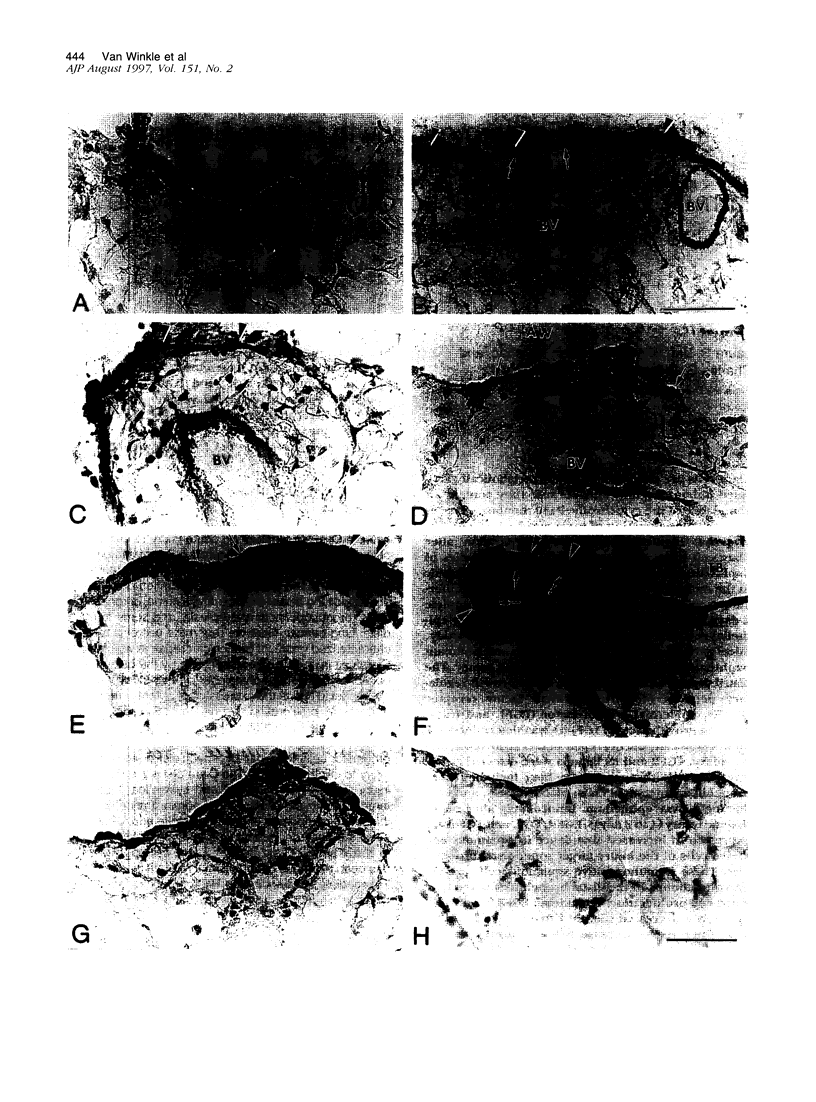
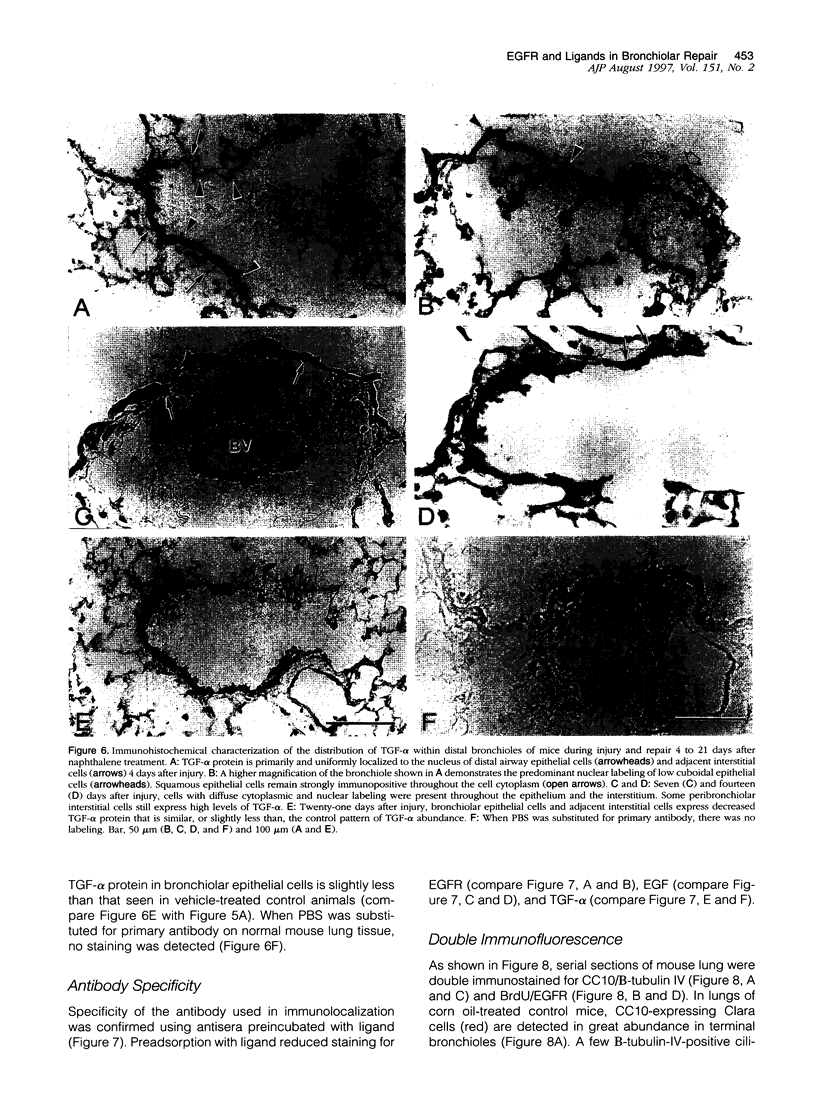
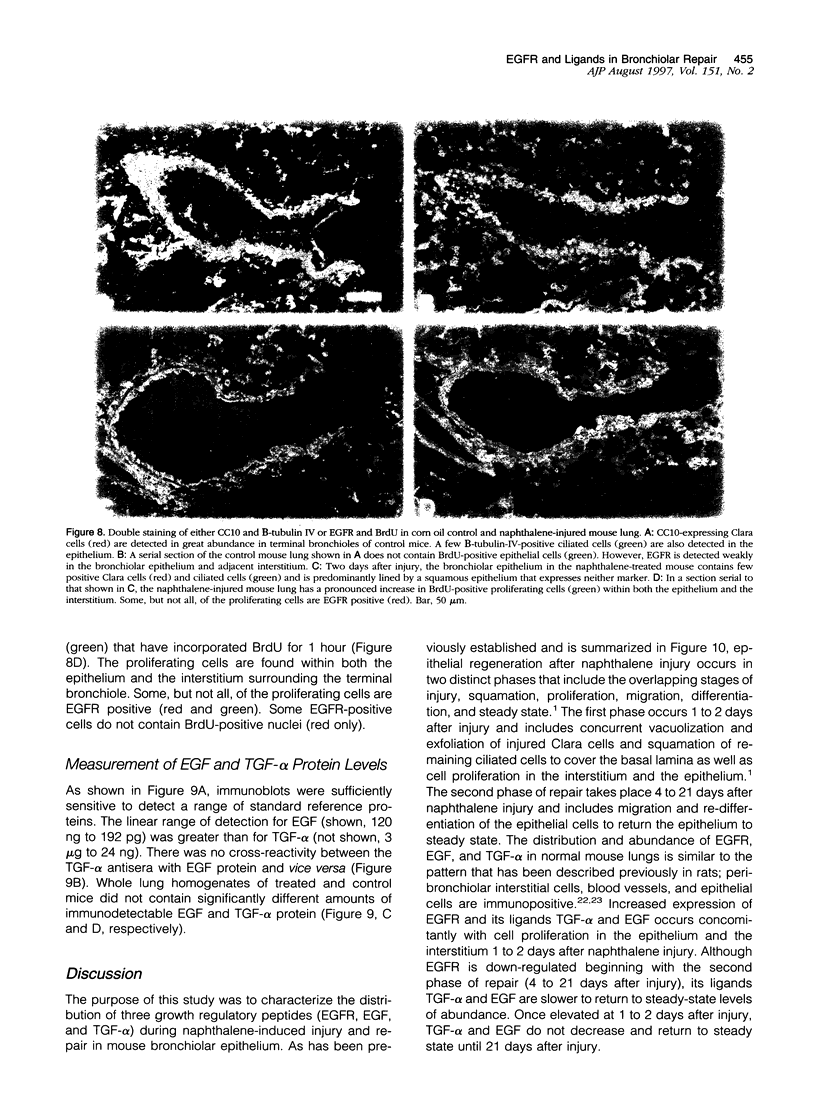
Clara cells are primary targets for metabolically activated pulmonary toxicants because they contain an abundance of the cytochrome P450 monooxygenases required for generation of toxic metabolites. The factors that regulate bronchiolar regeneration after Clara cell injury are not known. Previous studies of naphthalene-induced bronchiolar injury and repair in the mouse have shown that epithelial cell proliferation is maximal 1 to 2 days after injury and complete 4 days after injury. Proliferation is followed by epithelial re-differentiation (4 to 14 days). In this study, mice were treated with the environmental pollutant naphthalene to induce massive Clara cell injury. The distribution and abundance of three growth-regulatory peptides (epidermal growth factor receptor (EGFR), epidermal growth factor (EGF), and transforming growth factor (TGF)-alpha) was determined immunochemically during repair of this acute bronchiolar injury. EGFR and its ligands were detected at low levels in cells throughout the lung including peribronchiolar interstitial cells, blood vessels, and conducting airway epithelium. Immediately after naphthalene injury (1 to 2 days), EGFR, EGF, and TGF-alpha are expressed in increased abundance in squamous epithelial cells of the injury target zone, distal bronchioles. These immunopositive squamous cells are detected in clumps in the distal bronchioles at the time when cell proliferation is maximal. EGFR protein expression is decreased slightly 4 to 7 days after injury and continues to decrease below control levels of abundance 14 to 21 days after injury. This down-regulation of EGFR is not reflected in a corresponding decrease in EGF and TGF-alpha protein expression, indicating that control of cell proliferation is regulated at the receptor level. Co-localization of EGFR and bromodeoxyuridine-positive proliferating cells in the same bronchiole indicates that EGFR is up-regulated within the proliferative microenvironment as well as in specific proliferating cells within the injury target zone. The coincident localization within terminal bronchioles of EGFR, EGF, and TGF-alpha to groups of squamous epithelial cells 2 days after naphthalene injury suggests that these peptides are important in up-regulating cell proliferation after Clara cell injury in the mouse.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rubin J. S., Finch P. W., Wong J., Marchese C., Falco J., Taylor W. G., Kraus M. H. Growth factor-regulated pathways in epithelial cell proliferation. Am Rev Respir Dis. 1990 Dec;142(6 Pt 2):S7–10. doi: 10.1164/ajrccm/142.6_Pt_2.S7. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Hedgecock C., Bowden D. H. Epithelial cell-fibroblast interactions in lung injury and repair. Am J Pathol. 1990 Aug;137(2):385–392. [PMC free article] [PubMed] [Google Scholar]
- Aida S., Tamai S., Sekiguchi S., Shimizu N. Distribution of epidermal growth factor and epidermal growth factor receptor in human lung: immunohistochemical and immunoelectron-microscopic studies. Respiration. 1994;61(3):161–166. doi: 10.1159/000196329. [DOI] [PubMed] [Google Scholar]
- Baron J., Burke J. P., Guengerich F. P., Jakoby W. B., Voigt J. M. Sites for xenobiotic activation and detoxication within the respiratory tract: implications for chemically induced toxicity. Toxicol Appl Pharmacol. 1988 May;93(3):493–505. doi: 10.1016/0041-008x(88)90053-1. [DOI] [PubMed] [Google Scholar]
- Barr B. C., Hyde D. M., Plopper C. G., Dungworth D. L. Distal airway remodeling in rats chronically exposed to ozone. Am Rev Respir Dis. 1988 Apr;137(4):924–938. doi: 10.1164/ajrccm/137.4.924. [DOI] [PubMed] [Google Scholar]
- Boyd M. R. Evidence for the Clara cell as a site of cytochrome P450-dependent mixed-function oxidase activity in lung. Nature. 1977 Oct 20;269(5630):713–715. doi: 10.1038/269713a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buckpitt A., Buonarati M., Avey L. B., Chang A. M., Morin D., Plopper C. G. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. II. Comparison of stereoselectivity of naphthalene epoxidation in lung and nasal mucosa of mouse, hamster, rat and rhesus monkey. J Pharmacol Exp Ther. 1992 Apr;261(1):364–372. [PubMed] [Google Scholar]
- Buckpitt A., Chang A. M., Weir A., Van Winkle L., Duan X., Philpot R., Plopper C. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Mol Pharmacol. 1995 Jan;47(1):74–81. [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Schwartz L. W., Tyler W. S. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol. 1980 Mar;98(3):811–840. [PMC free article] [PubMed] [Google Scholar]
- Cohen S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev Biol. 1965 Dec;12(3):394–407. doi: 10.1016/0012-1606(65)90005-9. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral-Anderson L. J., Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978 Jun;38(6):648–653. [PubMed] [Google Scholar]
- Ferriola P. C., Earp H. S., Di Augustine R., Nettesheim P. Role of TGF alpha and its receptor in proliferation of immortalized rat tracheal epithelial cells: studies with tyrphostin and TGF alpha antisera. J Cell Physiol. 1991 Apr;147(1):166–175. doi: 10.1002/jcp.1041470121. [DOI] [PubMed] [Google Scholar]
- Kumar R. K., O'Grady R., Li W., Smith L. W., Rhodes G. C. Primary culture of adult mouse lung fibroblasts in serum-free medium: responses to growth factors. Exp Cell Res. 1991 Apr;193(2):398–404. doi: 10.1016/0014-4827(91)90112-8. [DOI] [PubMed] [Google Scholar]
- Lechner J. F., Haugen A., Autrup H., McClendon I. A., Trump B. F., Harris C. C. Clonal growth of epithelial cells from normal adult human bronchus. Cancer Res. 1981 Jun;41(6):2294–2304. [PubMed] [Google Scholar]
- Liu J. Y., Morris G. F., Lei W. H., Corti M., Brody A. R. Up-regulated expression of transforming growth factor-alpha in the bronchiolar-alveolar duct regions of asbestos-exposed rats. Am J Pathol. 1996 Jul;149(1):205–217. [PMC free article] [PubMed] [Google Scholar]
- Lum H., Schwartz L. W., Dungworth D. L., Tyler W. S. A comparative study of cell renewal after exposure to ozone or oxygen. Response of terminal bronchiolar epithelium in the rat. Am Rev Respir Dis. 1978 Aug;118(2):335–345. doi: 10.1164/arrd.1978.118.2.335. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Busby H. K., Strandjord T. P., Clark J. G. Expression of transforming growth factor-alpha and epidermal growth factor receptor is increased following bleomycin-induced lung injury in rats. Am J Respir Cell Mol Biol. 1994 Nov;11(5):540–551. doi: 10.1165/ajrcmb.11.5.7524566. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Malden L. T., Raines E. W., Ross R. Induction of transcription and secretion of TGF-alpha by activated human monocytes. Chest. 1991 Mar;99(3 Suppl):79S–79S. doi: 10.1378/chest.99.3_supplement.79s. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Ueda M., Ando N., Abe O., Shimizu N. Epidermal growth factor receptors in cancer tissues of esophagus, lung, pancreas, colorectum, breast and stomach. Jpn J Cancer Res. 1988 Nov;79(11):1201–1207. doi: 10.1111/j.1349-7006.1988.tb01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen A. M. Epidermal growth factor and transforming growth factor-alpha in the development of epithelial-mesenchymal organs of the mouse. Curr Top Dev Biol. 1990;24:31–55. [PubMed] [Google Scholar]
- Plopper C. G., Macklin J., Nishio S. J., Hyde D. M., Buckpitt A. R. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. III. Morphometric comparison of changes in the epithelial populations of terminal bronchioles and lobar bronchi in mice, hamsters, and rats after parenteral administration of naphthalene. Lab Invest. 1992 Nov;67(5):553–565. [PubMed] [Google Scholar]
- Plopper C. G., Suverkropp C., Morin D., Nishio S., Buckpitt A. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J Pharmacol Exp Ther. 1992 Apr;261(1):353–363. [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Serabjit-Singh C. J., Nishio S. J., Philpot R. M., Plopper C. G. The distribution of cytochrome P-450 monooxygenase in cells of the rabbit lung: an ultrastructural immunocytochemical characterization. Mol Pharmacol. 1988 Mar;33(3):279–289. [PubMed] [Google Scholar]
- Strandjord T. P., Clark J. G., Madtes D. K. Expression of TGF-alpha, EGF, and EGF receptor in fetal rat lung. Am J Physiol. 1994 Oct;267(4 Pt 1):L384–L389. doi: 10.1152/ajplung.1994.267.4.L384. [DOI] [PubMed] [Google Scholar]
- Stripp B. R., Maxson K., Mera R., Singh G. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995 Dec;269(6 Pt 1):L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- Thomassen D. G., Saffiotti U., Kaighn M. E. Clonal proliferation of rat tracheal epithelial cells in serum-free medium and their responses to hormones, growth factors and carcinogens. Carcinogenesis. 1986 Dec;7(12):2033–2039. doi: 10.1093/carcin/7.12.2033. [DOI] [PubMed] [Google Scholar]
- Turksen K., Choi Y., Fuchs E. Transforming growth factor alpha induces collagen degradation and cell migration in differentiating human epidermal raft cultures. Cell Regul. 1991 Aug;2(8):613–625. doi: 10.1091/mbc.2.8.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Winkle L. S., Buckpitt A. R., Nishio S. J., Isaac J. M., Plopper C. G. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol. 1995 Dec;269(6 Pt 1):L800–L818. doi: 10.1152/ajplung.1995.269.6.L800. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. S., Buckpitt A. R., Plopper C. G. Maintenance of differentiated murine Clara cells in microdissected airway cultures. Am J Respir Cell Mol Biol. 1996 Jun;14(6):586–598. doi: 10.1165/ajrcmb.14.6.8652187. [DOI] [PubMed] [Google Scholar]
- Vivekananda J., Lin A., Coalson J. J., King R. J. Acute inflammatory injury in the lung precipitated by oxidant stress induces fibroblasts to synthesize and release transforming growth factor-alpha. J Biol Chem. 1994 Oct 7;269(40):25057–25061. [PubMed] [Google Scholar]
- Wu R., Nolan E., Turner C. Expression of tracheal differentiated functions in serum-free hormone-supplemented medium. J Cell Physiol. 1985 Nov;125(2):167–181. doi: 10.1002/jcp.1041250202. [DOI] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985 Aug;132(2):311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- Yeh J., Yeh Y. C. Transforming growth factor-alpha and human cancer. Biomed Pharmacother. 1989;43(9):651–659. doi: 10.1016/0753-3322(89)90083-8. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]