Abstract
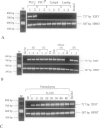
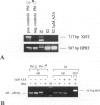
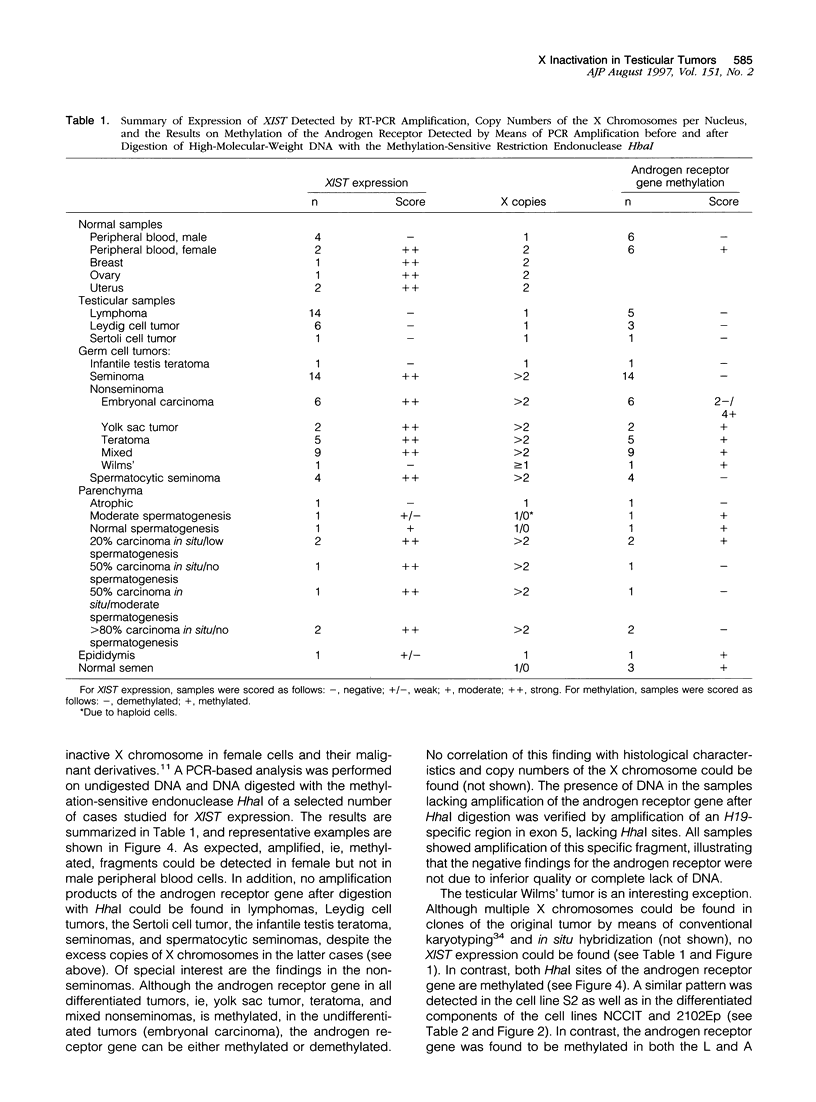
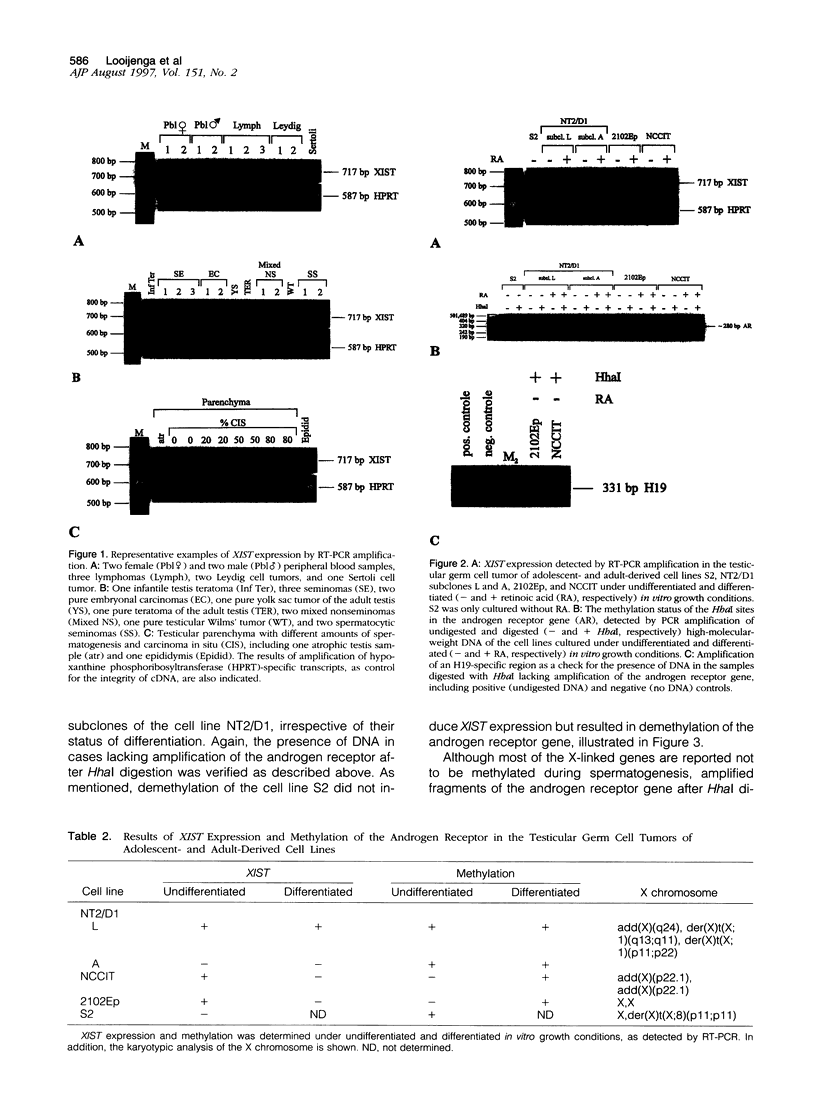
In female mammalian cells, inactivation of one of the X chromosomes compensates the increased dosage of X-linked genes as compared with their male counterparts. This process is initiated by the X-inactive specific transcripts of the xist/XIST gene in cis, resulting in methylation of specific sites of genes to be silenced. However, in male germ cells, X inactivation is established by xist/XIST expression only. We investigated the X inactivation pattern in human testicular tumors of different histogenesis by analysis of XIST expression and methylation of the androgen receptor gene. XIST was expressed only in tumors derived from the germ cell lineage with supernumerical X chromosomes: seminomas, nonseminomas, and spermatocytic seminomas. Although low expression was present in testicular parenchyma with spermatogenesis, XIST was expressed at a higher level in parenchyma with carcinoma in situ, the precursor lesion of seminomas and nonseminomas. Despite the consistent expression of XIST in germ-cell-derived tumors with gain of X chromosomes, methylation of the androgen receptor gene was present in all differentiated but only in a proportion of the undifferentiated nonseminomas. This differential pattern of methylation was also found in a number of representative cell lines. Our data indicate that the counting mechanism resulting in X inactivation is functional in testicular cancers of different histogenesis. Moreover, the differentiation-dependent pattern of X inactivation as reported during normal development in the case of multiple X chromosomes by methylation is retained in these tumors. We conclude therefore that X inactivation allows the excessive gain of X chromosomes found in germ-cell-derived tumors of the adult testis. In addition, this offers an interesting model to study the fundamental mechanisms of these processes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Casper J., Damjanov I., Duggan-Keen M., Giwercman A., Hata J., von Keitz A., Looijenga L. H., Millán J. L., Oosterhuis J. W. Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int J Cancer. 1996 Jun 11;66(6):806–816. doi: 10.1002/(SICI)1097-0215(19960611)66:6<806::AID-IJC17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Damjanov I., Simon D., Dignazio M. A pluripotent human stem-cell clone isolated from the TERA-2 teratocarcinoma line lacks antigens SSEA-3 and SSEA-4 in vitro, but expresses these antigens when grown as a xenograft tumor. Differentiation. 1985;29(2):127–135. doi: 10.1111/j.1432-0436.1985.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Andrews P. W., Nudelman E., Hakomori S., Fenderson B. A. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamethylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR). Differentiation. 1990 Apr;43(2):131–138. doi: 10.1111/j.1432-0436.1990.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Ariel M., Cedar H., McCarrey J. Developmental changes in methylation of spermatogenesis-specific genes include reprogramming in the epididymis. Nat Genet. 1994 May;7(1):59–63. doi: 10.1038/ng0594-59. [DOI] [PubMed] [Google Scholar]
- Atkin N. B., Baker M. C. X-chromatin, sex chromosomes, and ploidy in 37 germ cell tumors of the testis. Cancer Genet Cytogenet. 1992 Mar;59(1):54–56. doi: 10.1016/0165-4608(92)90158-5. [DOI] [PubMed] [Google Scholar]
- Ballabio A., Willard H. F. Mammalian X-chromosome inactivation and the XIST gene. Curr Opin Genet Dev. 1992 Jun;2(3):439–447. doi: 10.1016/s0959-437x(05)80155-8. [DOI] [PubMed] [Google Scholar]
- Beard C., Li E., Jaenisch R. Loss of methylation activates Xist in somatic but not in embryonic cells. Genes Dev. 1995 Oct 1;9(19):2325–2334. doi: 10.1101/gad.9.19.2325. [DOI] [PubMed] [Google Scholar]
- Borsani G., Tonlorenzi R., Simmler M. C., Dandolo L., Arnaud D., Capra V., Grompe M., Pizzuti A., Muzny D., Lawrence C. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991 May 23;351(6324):325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., McCabe V. M., Norris D. P., Cooper P. J., Swift S., Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992 Oct 30;71(3):515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Lafreniere R. G., Powers V. E., Sebastio G., Ballabio A., Pettigrew A. L., Ledbetter D. H., Levy E., Craig I. W., Willard H. F. Localization of the X inactivation centre on the human X chromosome in Xq13. Nature. 1991 Jan 3;349(6304):82–84. doi: 10.1038/349082a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Willard H. F. The human X-inactivation centre is not required for maintenance of X-chromosome inactivation. Nature. 1994 Mar 10;368(6467):154–156. doi: 10.1038/368154a0. [DOI] [PubMed] [Google Scholar]
- Carrel L., Clemson C. M., Dunn J. M., Miller A. P., Hunt P. A., Lawrence J. B., Willard H. F. X inactivation analysis and DNA methylation studies of the ubiquitin activating enzyme E1 and PCTAIRE-1 genes in human and mouse. Hum Mol Genet. 1996 Mar;5(3):391–401. doi: 10.1093/hmg/5.3.391. [DOI] [PubMed] [Google Scholar]
- Clemson C. M., McNeil J. A., Willard H. F., Lawrence J. B. XIST RNA paints the inactive X chromosome at interphase: evidence for a novel RNA involved in nuclear/chromosome structure. J Cell Biol. 1996 Feb;132(3):259–275. doi: 10.1083/jcb.132.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings O. W., Ulbright T. M., Eble J. N., Roth L. M. Spermatocytic seminoma: an immunohistochemical study. Hum Pathol. 1994 Jan;25(1):54–59. doi: 10.1016/0046-8177(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Horvat B., Gibas Z. Retinoic acid-induced differentiation of the developmentally pluripotent human germ cell tumor-derived cell line, NCCIT. Lab Invest. 1993 Feb;68(2):220–232. [PubMed] [Google Scholar]
- Gibbs R. A., Nguyen P. N., McBride L. J., Koepf S. M., Caskey C. T. Identification of mutations leading to the Lesch-Nyhan syndrome by automated direct DNA sequencing of in vitro amplified cDNA. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1919–1923. doi: 10.1073/pnas.86.6.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis A. J., Looijenga L. H., de Jong B., Oosterhuis J. W. Clonality of combined testicular germ cell tumors of adults. Lab Invest. 1994 Dec;71(6):874–878. [PubMed] [Google Scholar]
- Gillis A. J., Oosterhuis J. W., Schipper M. E., Barten E. J., van Berlo R., van Gurp R. J., Abraham M., Saunders G. F., Looijenga L. H. Origin and biology of a testicular Wilms' tumor. Genes Chromosomes Cancer. 1994 Oct;11(2):126–135. doi: 10.1002/gcc.2870110209. [DOI] [PubMed] [Google Scholar]
- Grant M., Zuccotti M., Monk M. Methylation of CpG sites of two X-linked genes coincides with X-inactivation in the female mouse embryo but not in the germ line. Nat Genet. 1992 Oct;2(2):161–166. doi: 10.1038/ng1092-161. [DOI] [PubMed] [Google Scholar]
- Hendrich B. D., Brown C. J., Willard H. F. Evolutionary conservation of possible functional domains of the human and murine XIST genes. Hum Mol Genet. 1993 Jun;2(6):663–672. doi: 10.1093/hmg/2.6.663. [DOI] [PubMed] [Google Scholar]
- Hittmair A., Rogatsch H., Feichtinger H., Hobisch A., Mikuz G. Carcinoma in situ of the testis detected by DNA flow cytometry of testicular fine-needle aspirates. Cytometry. 1994 Dec 1;17(4):327–331. doi: 10.1002/cyto.990170408. [DOI] [PubMed] [Google Scholar]
- Hittmair A., Rogatsch H., Feichtinger H., Hobisch A., Mikuz G. Testicular seminomas are aneuploid tumors. Lab Invest. 1995 Jan;72(1):70–74. [PubMed] [Google Scholar]
- Jørgensen N., Rajpert-De Meyts E., Graem N., Müller J., Giwercman A., Skakkebaek N. E. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest. 1995 Feb;72(2):223–231. [PubMed] [Google Scholar]
- Kysela B., Matoska J. Flow cytometry analysis of ploidy and proliferation activity in classical and spermatocytic seminoma. Neoplasma. 1991;38(1):3–11. [PubMed] [Google Scholar]
- Lee J. T., Strauss W. M., Dausman J. A., Jaenisch R. A 450 kb transgene displays properties of the mammalian X-inactivation center. Cell. 1996 Jul 12;86(1):83–94. doi: 10.1016/s0092-8674(00)80079-3. [DOI] [PubMed] [Google Scholar]
- Looijenga L. H., Gillis A. J., Van Putten W. L., Oosterhuis J. W. In situ numeric analysis of centromeric regions of chromosomes 1, 12, and 15 of seminomas, nonseminomatous germ cell tumors, and carcinoma in situ of human testis. Lab Invest. 1993 Feb;68(2):211–219. [PubMed] [Google Scholar]
- Looijenga L. H., Olie R. A., van der Gaag I., van Sluijs F. J., Matoska J., Ploem-Zaaijer J., Knepflé C., Oosterhuis J. W. Seminomas of the canine testis. Counterpart of spermatocytic seminoma of men? Lab Invest. 1994 Oct;71(4):490–496. [PubMed] [Google Scholar]
- Lyon M. F. Some milestones in the history of X-chromosome inactivation. Annu Rev Genet. 1992;26:16–28. doi: 10.1146/annurev.ge.26.120192.000313. [DOI] [PubMed] [Google Scholar]
- McCarrey J. R., Dilworth D. D. Expression of Xist in mouse germ cells correlates with X-chromosome inactivation. Nat Genet. 1992 Nov;2(3):200–203. doi: 10.1038/ng1192-200. [DOI] [PubMed] [Google Scholar]
- Mosselman S., Looijenga L. H., Gillis A. J., van Rooijen M. A., Kraft H. J., van Zoelen E. J., Oosterhuis J. W. Aberrant platelet-derived growth factor alpha-receptor transcript as a diagnostic marker for early human germ cell tumors of the adult testis. Proc Natl Acad Sci U S A. 1996 Apr 2;93(7):2884–2888. doi: 10.1073/pnas.93.7.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert M. M., van de Pol M., Olde Weghuis D., Suijkerbuijk R. F., Geurts van Kessel A., van Echten J., Oosterhuis J. W., Looijenga L. H. Comparative genomic hybridization of germ cell tumors of the adult testis: confirmation of karyotypic findings and identification of a 12p-amplicon. Cancer Genet Cytogenet. 1996 Jul 15;89(2):146–152. doi: 10.1016/0165-4608(96)00043-x. [DOI] [PubMed] [Google Scholar]
- Mostert M. M., van de Pol M., van Echten J., Olde Weghuis D., Geurts van Kessel A., Oosterhuis J. W., Looijenga L. H. Fluorescence in situ hybridization-based approaches for detection of 12p overrepresentation, in particular i(12p), in cell lines of human testicular germ cell tumors of adults. Cancer Genet Cytogenet. 1996 Apr;87(2):95–102. doi: 10.1016/0165-4608(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Mostofi F. K., Sesterhenn I. A., Davis C. J., Jr Immunopathology of germ cell tumors of the testis. Semin Diagn Pathol. 1987 Nov;4(4):320–341. [PubMed] [Google Scholar]
- Mutter G. L., Boynton K. A. X chromosome inactivation in the normal female genital tract: implications for identification of neoplasia. Cancer Res. 1995 Nov 1;55(21):5080–5084. [PubMed] [Google Scholar]
- Mutter G. L., Chaponot M. L., Fletcher J. A. A polymerase chain reaction assay for non-random X chromosome inactivation identifies monoclonal endometrial cancers and precancers. Am J Pathol. 1995 Feb;146(2):501–508. [PMC free article] [PubMed] [Google Scholar]
- Nakashima N., Murakami S., Fukatsu T., Nagasaka T., Fukata S., Ohiwa N., Nara Y., Sobue M., Takeuchi J. Characteristics of "embryoid body" in human gonadal germ cell tumors. Hum Pathol. 1988 Oct;19(10):1144–1154. doi: 10.1016/s0046-8177(88)80145-x. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas: summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer. 1982 May 15;49(10):2112–2135. doi: 10.1002/1097-0142(19820515)49:10<2112::aid-cncr2820491024>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Norris D. P., Patel D., Kay G. F., Penny G. D., Brockdorff N., Sheardown S. A., Rastan S. Evidence that random and imprinted Xist expression is controlled by preemptive methylation. Cell. 1994 Apr 8;77(1):41–51. doi: 10.1016/0092-8674(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Oosterhuis J. W., Castedo S. M., de Jong B., Cornelisse C. J., Dam A., Sleijfer D. T., Schraffordt Koops H. Ploidy of primary germ cell tumors of the testis. Pathogenetic and clinical relevance. Lab Invest. 1989 Jan;60(1):14–21. [PubMed] [Google Scholar]
- Oosterhuis J. W., Looijenga L. H. The biology of human germ cell tumours: retrospective speculations and new prospectives. Eur Urol. 1993;23(1):245–250. doi: 10.1159/000474601. [DOI] [PubMed] [Google Scholar]
- Panning B., Jaenisch R. DNA hypomethylation can activate Xist expression and silence X-linked genes. Genes Dev. 1996 Aug 15;10(16):1991–2002. doi: 10.1101/gad.10.16.1991. [DOI] [PubMed] [Google Scholar]
- Peltomäki P. DNA methylation changes in human testicular cancer. Biochim Biophys Acta. 1991 Apr 15;1096(3):187–196. doi: 10.1016/0925-4439(91)90004-s. [DOI] [PubMed] [Google Scholar]
- Penny G. D., Kay G. F., Sheardown S. A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996 Jan 11;379(6561):131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Richler C., Soreq H., Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992 Nov;2(3):192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Roach S., Cooper S., Bennett W., Pera M. F. Cultured cell lines from human teratomas: windows into tumour growth and differentiation and early human development. Eur Urol. 1993;23(1):82–88. doi: 10.1159/000474574. [DOI] [PubMed] [Google Scholar]
- Salido E. C., Yen P. H., Mohandas T. K., Shapiro L. J. Expression of the X-inactivation-associated gene XIST during spermatogenesis. Nat Genet. 1992 Nov;2(3):196–199. doi: 10.1038/ng1192-196. [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E., Berthelsen J. G., Giwercman A., Müller J. Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl. 1987 Feb;10(1):19–28. doi: 10.1111/j.1365-2605.1987.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Tai H. H., Gordon J., McBurney M. W. Xist is expressed in female embryonal carcinoma cells with two active X chromosomes. Somat Cell Mol Genet. 1994 May;20(3):171–182. doi: 10.1007/BF02254758. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Ariel I., Dekker M. C., Schneider T., van Gurp R. J., de Groot N., Gillis A. J., Oosterhuis J. W., Hochberg A. A., Looijenga L. H. Unique expression patterns of H19 in human testicular cancers of different etiology. Oncogene. 1997 Jan 9;14(1):95–107. doi: 10.1038/sj.onc.1200802. [DOI] [PubMed] [Google Scholar]
- Walt H., Oosterhuis J. W., Stevens L. C. Experimental testicular germ cell tumorigenesis in mouse strains with and without spontaneous tumours differs from development of germ cell tumours of the adult human testis. Int J Androl. 1993 Aug;16(4):267–271. doi: 10.1111/j.1365-2605.1993.tb01190.x. [DOI] [PubMed] [Google Scholar]
- Wang N., Perkins K. L., Bronson D. L., Fraley E. E. Cytogenetic evidence for premeiotic transformation of human testicular cancers. Cancer Res. 1981 Jun;41(6):2135–2140. [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard H. F. X chromosome inactivation, XIST, and pursuit of the X-inactivation center. Cell. 1996 Jul 12;86(1):5–7. doi: 10.1016/s0092-8674(00)80071-9. [DOI] [PubMed] [Google Scholar]
- Zuccotti M., Monk M. Methylation of the mouse Xist gene in sperm and eggs correlates with imprinted Xist expression and paternal X-inactivation. Nat Genet. 1995 Mar;9(3):316–320. doi: 10.1038/ng0395-316. [DOI] [PubMed] [Google Scholar]
- el-Naggar A. K., Ro J. Y., McLemore D., Ayala A. G., Batsakis J. G. DNA ploidy in testicular germ cell neoplasms. Histogenetic and clinical implications. Am J Surg Pathol. 1992 Jun;16(6):611–618. doi: 10.1097/00000478-199206000-00009. [DOI] [PubMed] [Google Scholar]
- van Echten J., Oosterhuis J. W., Looijenga L. H., van de Pol M., Wiersema J., te Meerman G. J., Schaffordt Koops H., Sleijfer D. T., de Jong B. No recurrent structural abnormalities apart from i(12p) in primary germ cell tumors of the adult testis. Genes Chromosomes Cancer. 1995 Oct;14(2):133–144. doi: 10.1002/gcc.2870140208. [DOI] [PubMed] [Google Scholar]
- von Keitz A. T., Riedmiller H., Neumann K., Gutschank W., Fonatsch C. Establishment and characterization of a seminoma cell-line (S2). Investig Urol (Berl) 1994;5:28–31. [PubMed] [Google Scholar]