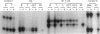Abstract
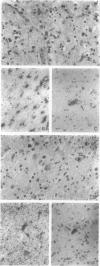
Gangliogliomas are rare tumors of the central nervous system that account for approximately 1% of all brain tumors. Histologically, gangliogliomas are composed of intimately admixed glial and neuronal components, the pathological origins of which remain to be characterized. Clonal analysis through examination of the pattern of the X chromosome inactivation allows one to distinguish monoclonal differentiation of a genetically abnormal progenitor cell from parallel, but independent, clonal expansion of two different cell types during tumorigenesis in biphasic neoplasms, such as gangliogliomas. In the present study, we investigated the clonality of eight gangliogliomas from female patients using both methylation- and transcription-based clonality assays at the androgen receptor locus (HUMARA) on the X chromosome. Among tumors from seven patients who were heterozygous at the HUMARA locus, five were identified as monoclonal with the methylation-based clonality assay, and the results were confirmed by the transcription-based method, whereas two were shown to be polyclonal by the methylation-based clonality assay but monoclonal by transcription-based clonality analysis. We conclude that the predominant cell types in most gangliogliomas are monoclonal in origin and derive from a common precursor cell that subsequently differentiates to form neoplastic glial and neuronal elements.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson G., Fraser N. J., Boyd J., Craig I., Wainscoat J. S. A highly informative X-chromosome probe, M27 beta, can be used for the determination of tumour clonality. Br J Haematol. 1990 Mar;74(3):371–372. doi: 10.1111/j.1365-2141.1990.tb02601.x. [DOI] [PubMed] [Google Scholar]
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Baylin S. B. Abnormal regional hypermethylation in cancer cells. AIDS Res Hum Retroviruses. 1992 May;8(5):811–820. [PubMed] [Google Scholar]
- Baylin S. B., Makos M., Wu J. J., Yen R. W., de Bustros A., Vertino P., Nelkin B. D. Abnormal patterns of DNA methylation in human neoplasia: potential consequences for tumor progression. Cancer Cells. 1991 Oct;3(10):383–390. [PubMed] [Google Scholar]
- Bowles A. P., Jr, Pantazis C. G., Allen M. B., Jr, Martinez J., Allsbrook W. C., Jr Ganglioglioma, a malignant tumor? Correlation with flow deoxyribonucleic acid cytometric analysis. Neurosurgery. 1988 Sep;23(3):376–381. doi: 10.1227/00006123-198809000-00019. [DOI] [PubMed] [Google Scholar]
- Busque L., Zhu J., DeHart D., Griffith B., Willman C., Carroll R., Black P. M., Gilliland D. G. An expression based clonality assay at the human androgen receptor locus (HUMARA) on chromosome X. Nucleic Acids Res. 1994 Feb 25;22(4):697–698. doi: 10.1093/nar/22.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Gartler S. M., Yoshida A. Clonal origin of chronic myelocytic leukemia in man. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1468–1471. doi: 10.1073/pnas.58.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow P. J. Use of genetic markers to study cellular origin and development of tumors in human females. Adv Cancer Res. 1972;15:191–226. doi: 10.1016/s0065-230x(08)60375-9. [DOI] [PubMed] [Google Scholar]
- Frederiksen K., Jat P. S., Valtz N., Levy D., McKay R. Immortalization of precursor cells from the mammalian CNS. Neuron. 1988 Aug;1(6):439–448. doi: 10.1016/0896-6273(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Gale R. E., Wheadon H., Linch D. C. Assessment of X-chromosome inactivation patterns using the hypervariable probe M27 beta in normal hemopoietic cells and acute myeloid leukemic blasts. Leukemia. 1992 Jul;6(7):649–655. [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Green A. J., Sepp T., Yates J. R. Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet. 1996 Feb;97(2):240–243. doi: 10.1007/BF02265273. [DOI] [PubMed] [Google Scholar]
- Hakim R., Loeffler J. S., Anthony D. C., Black P. M. Gangliogliomas in adults. Cancer. 1997 Jan 1;79(1):127–131. [PubMed] [Google Scholar]
- Hall W. A., Yunis E. J., Albright A. L. Anaplastic ganglioglioma in an infant: case report and review of the literature. Neurosurgery. 1986 Dec;19(6):1016–1020. doi: 10.1227/00006123-198612000-00019. [DOI] [PubMed] [Google Scholar]
- Jay V., Squire J., Becker L. E., Humphreys R. Malignant transformation in a ganglioglioma with anaplastic neuronal and astrocytic components. Report of a case with flow cytometric and cytogenetic analysis. Cancer. 1994 Jun 1;73(11):2862–2868. doi: 10.1002/1097-0142(19940601)73:11<2862::aid-cncr2820731133>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Johannsson J. H., Rekate H. L., Roessmann U. Gangliogliomas: pathological and clinical correlation. J Neurosurg. 1981 Jan;54(1):58–63. doi: 10.3171/jns.1981.54.1.0058. [DOI] [PubMed] [Google Scholar]
- Kondziolka D., Bilbao J. M. Mixed ependymoma-astrocytoma (subependymoma?) of the cerebral cortex. Acta Neuropathol. 1988;76(6):633–637. doi: 10.1007/BF00689604. [DOI] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sullivan P. M., Willard H. F., French F. S., Wilson E. M. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988 Apr 15;240(4850):327–330. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- Makos M., Nelkin B. D., Chazin V. R., Cavenee W. K., Brodeur G. M., Baylin S. B. DNA hypermethylation is associated with 17p allelic loss in neural tumors. Cancer Res. 1993 Jun 15;53(12):2715–2718. [PubMed] [Google Scholar]
- Mashal R. D., Lester S. C., Sklar J. Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue. Cancer Res. 1993 Oct 1;53(19):4676–4679. [PubMed] [Google Scholar]
- McKay R. D. The origins of cellular diversity in the mammalian central nervous system. Cell. 1989 Sep 8;58(5):815–821. doi: 10.1016/0092-8674(89)90934-3. [DOI] [PubMed] [Google Scholar]
- Miller D. C., Lang F. F., Epstein F. J. Central nervous system gangliogliomas. Part 1: Pathology. J Neurosurg. 1993 Dec;79(6):859–866. doi: 10.3171/jns.1993.79.6.0859. [DOI] [PubMed] [Google Scholar]
- Park S. H., Chi J. G., Cho B. K., Wang K. C. Spinal cord ganglioglioma in childhood. Pathol Res Pract. 1993 Mar;189(2):189–196. doi: 10.1016/S0344-0338(11)80091-9. [DOI] [PubMed] [Google Scholar]
- RUSSELL D. S., RUBINSTEIN L. J. Ganglioglioma: a case with long history and malignant evolution. J Neuropathol Exp Neurol. 1962 Apr;21:185–193. [PubMed] [Google Scholar]
- Reynolds B. A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992 Mar 27;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Sun Y., Hegamyer G., Colburn N. H. A simple method using PCR for direct sequencing of genomic DNA from frozen tumor tissue embedded in optimal cutting temperature compound. Biotechniques. 1992 May;12(5):639–640. [PubMed] [Google Scholar]
- Sutton L. N., Packer R. J., Rorke L. B., Bruce D. A., Schut L. Cerebral gangliogliomas during childhood. Neurosurgery. 1983 Aug;13(2):124–128. doi: 10.1227/00006123-198308000-00003. [DOI] [PubMed] [Google Scholar]
- Ventureyra E., Herder S., Mallya B. K., Keene D. Temporal lobe gangliogliomas in children. Childs Nerv Syst. 1986;2(2):63–66. doi: 10.1007/BF00286222. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Wainscoat J. S., Fey M. F. Assessment of clonality in human tumors: a review. Cancer Res. 1990 Mar 1;50(5):1355–1360. [PubMed] [Google Scholar]
- Willman C. L., Busque L., Griffith B. B., Favara B. E., McClain K. L., Duncan M. H., Gilliland D. G. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med. 1994 Jul 21;331(3):154–160. doi: 10.1056/NEJM199407213310303. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F. Tumor clonality and its biological significance. Adv Cancer Res. 1988;50:197–229. doi: 10.1016/s0065-230x(08)60438-8. [DOI] [PubMed] [Google Scholar]
- Zhu J., Frosch M. P., Busque L., Beggs A. H., Dashner K., Gilliland D. G., Black P. M. Analysis of meningiomas by methylation- and transcription-based clonality assays. Cancer Res. 1995 Sep 1;55(17):3865–3872. [PubMed] [Google Scholar]
- Zhu J., Leon S. P., Beggs A. H., Busque L., Gilliland D. G., Black P. M. Human pituitary adenomas show no loss of heterozygosity at the retinoblastoma gene locus. J Clin Endocrinol Metab. 1994 Apr;78(4):922–927. doi: 10.1210/jcem.78.4.8157722. [DOI] [PubMed] [Google Scholar]