Abstract
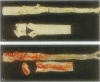
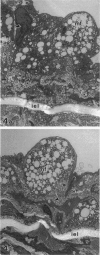
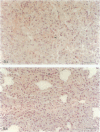
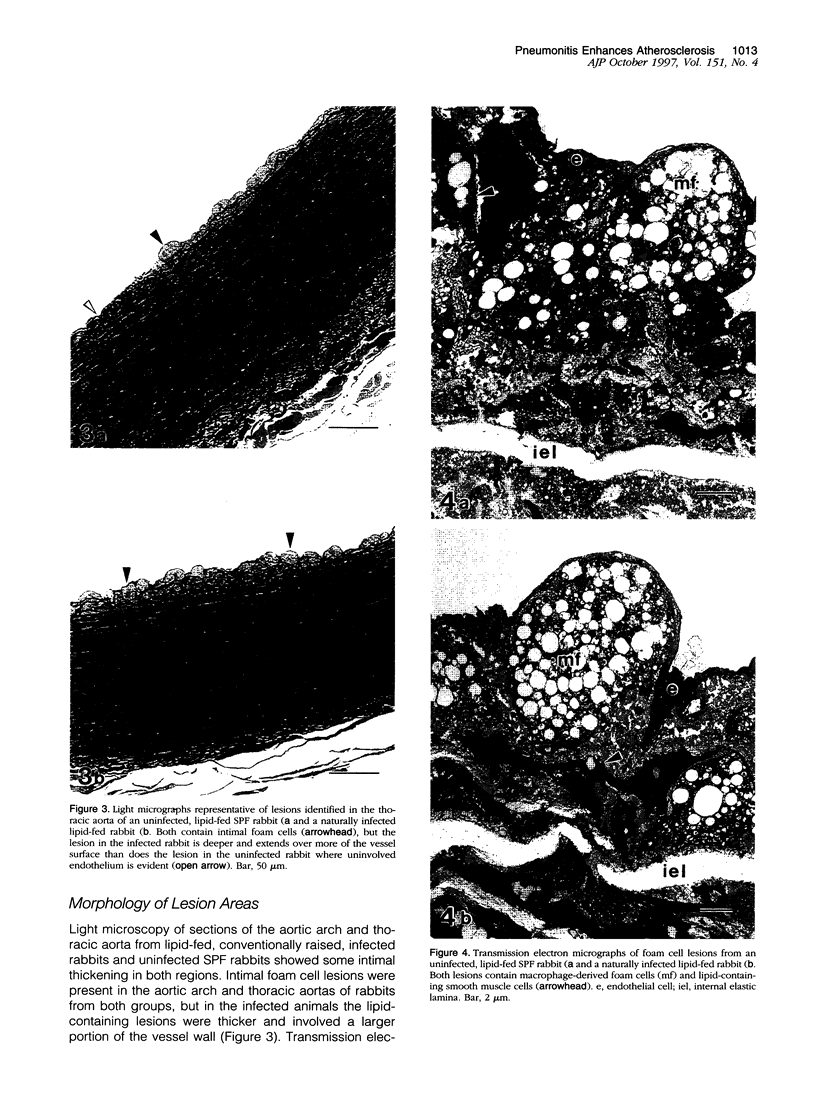
The pathogenesis of atherosclerosis has been related to infection of the arterial wall, but it is not clear whether this occurs before or after the development of lipid-containing lesions. Respiratory bacterial infection increases the expression of vascular cell adhesion molecule-1 (VCAM-1). We therefore examined whether a similar infection would enhance atherosclerosis in New Zealand White rabbits fed chow supplemented by 15% (w/w) egg yolk for 50 days. Rabbits with naturally acquired respiratory infection by Pasteurella multocida, pathogen-free (SPF) animals infected by P. multocida in the laboratory, and age-matched SPF rabbits maintained in a disease-free environment were used. Endothelial cells expressing VCAM-1 in the aorta between intercostal arteries 3 and 5 were identified using anti-VCAM-1 (Rb1/9) and an alkaline-phosphatase-linked secondary antibody and quantified in Häutchen preparations. The remainder of the aorta was stained with Sudan IV to show lipid deposition. The expression of VCAM-1 (mean +/- SEM per 10,000 cells) was 22 +/- 8 (n = 5) in the lipid-fed SPF rabbits, significantly different from that in the lipid-fed rabbits with naturally occurring infection (190 +/- 51 (n = 5)) or from rabbits infected in the laboratory (106 +/- 25 (n = 5)). The extent of Sudanophilia was significantly greater in the naturally infected rabbits (8.3 +/- 1.2%) or infected SPF rabbits (10.3 +/- 1.8%) than in the SPF rabbits (2.7 +/- 0.8%; P < 0.05). Antibiotic treatment in naturally infected rabbits reduced the number of cells expressing VCAM-1 and the extent of the Sudanophilia to baseline levels. Thus, Sudanophilia is enhanced by bacterial infection in rabbits fed egg yolk and is associated with a significant increase in VCAM-1.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavi M. Z., Galis Z., Li Z. H., Moore S. Dietary alterations of plasma lipoproteins influence their interactions with proteoglycan enriched extracts from neointima of normal and injured aorta of rabbit. Clin Invest Med. 1991 Oct;14(5):419–431. [PubMed] [Google Scholar]
- Alavi M. Z., Wasty F., Li Z., Galis Z. S., Ismail N., Moore S. Enhanced incorporation of [14C]glucosamine into glycosaminoglycans of aortic neointima of balloon-injured and cholesterol-fed rabbits in vitro. Atherosclerosis. 1992 Jul;95(1):59–67. doi: 10.1016/0021-9150(92)90176-h. [DOI] [PubMed] [Google Scholar]
- Ardehali A., Laks H., Drinkwater D. C., Ziv E., Drake T. A. Vascular cell adhesion molecule-1 is induced on vascular endothelia and medial smooth muscle cells in experimental cardiac allograft vasculopathy. Circulation. 1995 Aug 1;92(3):450–456. doi: 10.1161/01.cir.92.3.450. [DOI] [PubMed] [Google Scholar]
- Brankin B., Hart M. N., Cosby S. L., Fabry Z., Allen I. V. Adhesion molecule expression and lymphocyte adhesion to cerebral endothelium: effects of measles virus and herpes simplex 1 virus. J Neuroimmunol. 1995 Jan;56(1):1–8. doi: 10.1016/0165-5728(94)00110-a. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Yeung A. C., Schoen F. J., Allred E. N., Stavrakis G., Ganz P., Cotran R. S., Pober J. S., Schoen E. L. Predictive value of inducible endothelial cell adhesion molecule expression for acute rejection of human cardiac allografts. Transplantation. 1995 Jan 27;59(2):204–211. [PubMed] [Google Scholar]
- Bruggeman C. A., van Dam-Mieras M. C. The possible role of cytomegalovirus in atherogenesis. Prog Med Virol. 1991;38:1–26. [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Datta S. K., Tumilowicz J. J., Trentin J. J. Lysis of human arterial smooth muscle cells infected with herpesviridae by peripheral blood mononuclear cells: implications for atherosclerosis. Viral Immunol. 1993 Summer;6(2):153–160. doi: 10.1089/vim.1993.6.153. [DOI] [PubMed] [Google Scholar]
- Feldman D. L., Mogelesky T. C., Liptak B. F., Gerrity R. G. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991 Jul-Aug;11(4):985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Chiu B., Viira E., Fong M. W., Jang D., Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997 Jan;35(1):48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B., Falcone D. J., Weksler B. B., Grant A. J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J Clin Invest. 1987 Nov;80(5):1317–1321. doi: 10.1172/JCI113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hardin N. J., Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. 3. The role of earlier acquired fibromuscular intimal thickening in the pathogenesis of later developing atherosclerosis. Am J Pathol. 1973 Nov;73(2):301–326. [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol. 1994 Jan;68(1):1–5. doi: 10.1128/jvi.68.1.1-5.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B. V., Parthasarathy S. S., Alexander R. W., Medford R. M. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995 Mar;95(3):1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C. L., Köhler H., Bittinger F., Wagner M., Hermanns I., Grant K., Lewis J. C., Kirkpatrick C. J. Comparative studies on vascular endothelium in vitro. I. Cytokine effects on the expression of adhesion molecules by human umbilical vein, saphenous vein and femoral artery endothelial cells. Pathobiology. 1994;62(4):199–208. doi: 10.1159/000163911. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Shor A., Campbell L. A., Fukushi H., Patton D. L., Grayston J. T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993 Apr;167(4):841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- Lamberson H. V., Jr, Fritz K. E. Immunological enhancement of atherogenesis in rabbits. Persistent susceptibility to atherogenic diet following experimentally induced serum sickness. Arch Pathol. 1974 Jul;98(1):9–16. [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- MITCHELL J. R., SCHWARTZ C. J., ZINGER A. RELATIONSHIP BETWEEN AORTIC PLAQUES AND AGE, SEX, AND BLOOD PRESSURE. Br Med J. 1964 Jan 25;1(5377):205–209. doi: 10.1136/bmj.1.5377.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick J. L., Hu C., Burek J., Adam E., DeBakey M. E. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J Med Virol. 1994 Feb;42(2):170–174. doi: 10.1002/jmv.1890420213. [DOI] [PubMed] [Google Scholar]
- O'Brien K. D., Allen M. D., McDonald T. O., Chait A., Harlan J. M., Fishbein D., McCarty J., Ferguson M., Hudkins K., Benjamin C. D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993 Aug;92(2):945–951. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Richardson M., Hadcock S. J., DeReske M., Cybulsky M. I. Increased expression in vivo of VCAM-1 and E-selectin by the aortic endothelium of normolipemic and hyperlipemic diabetic rabbits. Arterioscler Thromb. 1994 May;14(5):760–769. doi: 10.1161/01.atv.14.5.760. [DOI] [PubMed] [Google Scholar]
- Ridker P. M., Cushman M., Stampfer M. J., Tracy R. P., Hennekens C. H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997 Apr 3;336(14):973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Ringler D. H., Peter G. K., Chrisp C. E., Keren D. F. Protection of rabbits against experimental pasteurellosis by vaccination with a potassium thiocyanate extract of Pasteurella multocida. Infect Immun. 1985 Sep;49(3):498–504. doi: 10.1128/iai.49.3.498-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sedmak D. D., Knight D. A., Vook N. C., Waldman J. W. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation. 1994 Dec 27;58(12):1379–1385. [PubMed] [Google Scholar]
- Sharma S. A., Olchowy T. W., Yang Z., Breider M. A. Tumor necrosis factor alpha and interleukin 1 alpha enhance lipopolysaccharide-mediated bovine endothelial cell injury. J Leukoc Biol. 1992 Jun;51(6):579–585. doi: 10.1002/jlb.51.6.579. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Sukhova G. K., Swanson S. J., Clinton S. K., Ganz P., Cybulsky M. I., Libby P. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993 Oct;88(4 Pt 1):1788–1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- Waldman W. J., Knight D. A. Cytokine-mediated induction of endothelial adhesion molecule and histocompatibility leukocyte antigen expression by cytomegalovirus-activated T cells. Am J Pathol. 1996 Jan;148(1):105–119. [PMC free article] [PubMed] [Google Scholar]
- Wissler R. W. Significance of Chlamydia pneumoniae (TWAR) in atherosclerotic lesions. Circulation. 1995 Dec 15;92(12):3376–3376. doi: 10.1161/01.cir.92.12.3376. [DOI] [PubMed] [Google Scholar]
- Wood K. M., Cadogan M. D., Ramshaw A. L., Parums D. V. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993 May;22(5):437–444. doi: 10.1111/j.1365-2559.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]