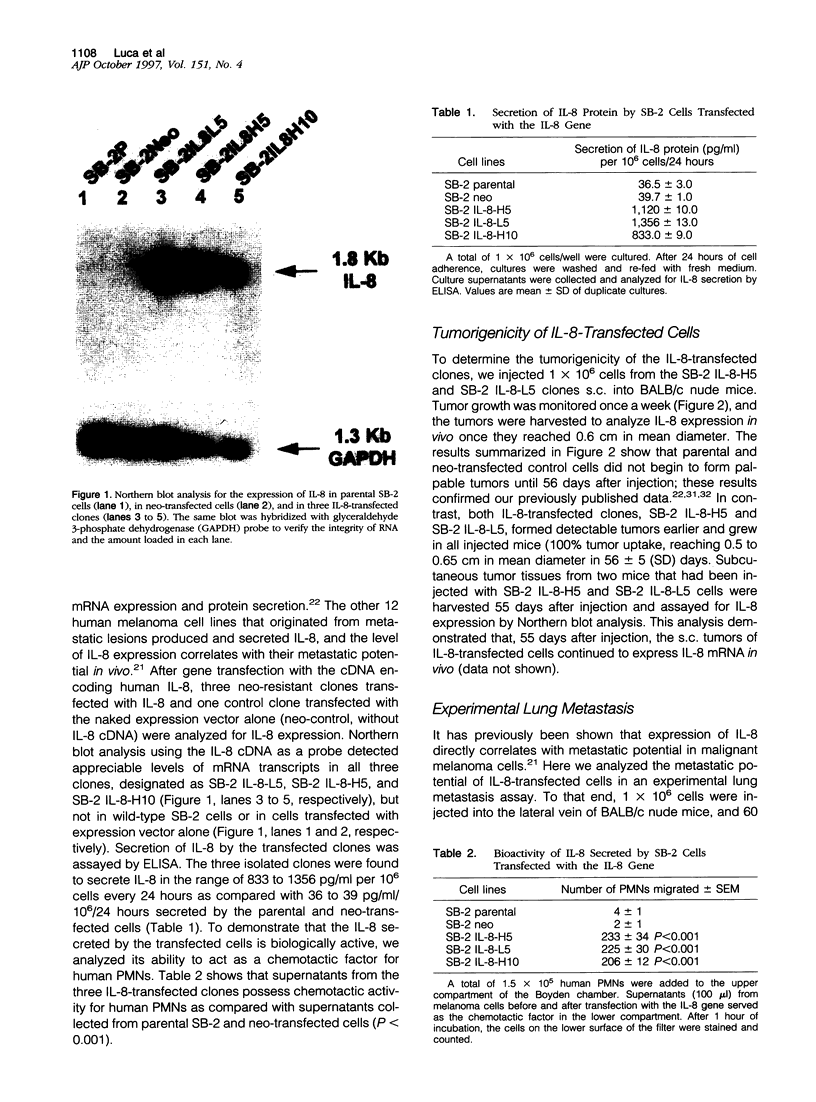
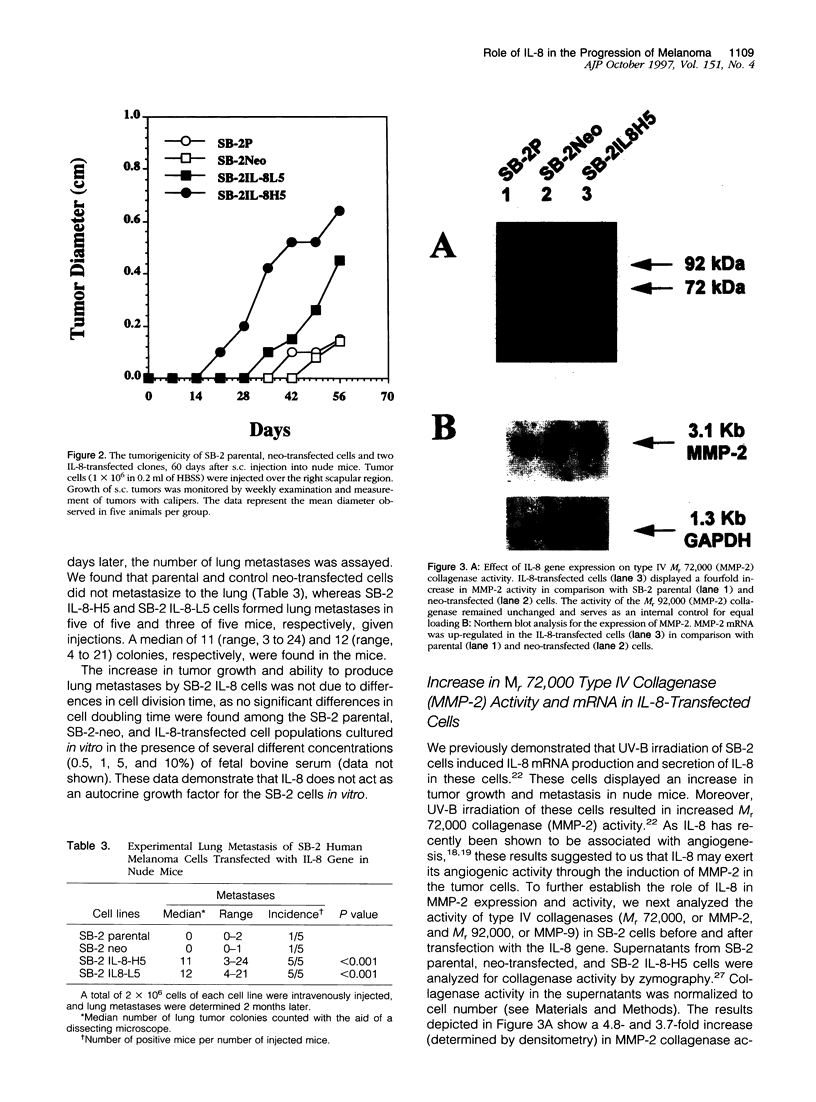
Abstract
Expression of interleukin-8 (IL-8) by human melanoma cells correlates with their metastatic potential. Moreover, UV-B irradiation of primary cutaneous melanoma cells induces IL-8 mRNA and protein production and increases both tumor growth and metastasis in nude mice. Although IL-8 has been shown to be an angiogenic factor, the biological consequences of increased IL-8 production by melanoma cells and the role of IL-8 in the metastatic process remains unclear. The purpose of this study was to determine the role of IL-8 in tumor growth and metastasis of human melanoma cells. Nonmetastatic SB-2 melanoma cells with negligible levels of IL-8 were transfected with IL-8 cDNA and subsequently analyzed for changes in their tumorigenic and metastatic potential. Enforced expression of IL-8 rendered the melanoma cells highly tumorigenic and increased their metastatic potential as compared with parental and control transfected cells. The IL-8-transfected cells displayed up-regulation in M(r) 72,000 collagenase type IV (MMP-2) mRNA and collagenase activity and increased invasiveness through Matrigel-coated filters. Moreover, when the MMP-2 promoter was linked upstream of the chloramphenicol acetyltransferase (CAT) reporter gene, CAT activity was up-regulated in IL-8 but not in control transfected cells, suggesting that IL-8 is involved in MMP-2 gene transcription. Activation of type IV collagenase by IL-8 can enhance the invasion of host stroma by the tumor cells and increase angiogenesis and, hence, metastasis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Anisowicz A., Zajchowski D., Stenman G., Sager R. Functional diversity of gro gene expression in human fibroblasts and mammary epithelial cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9645–9649. doi: 10.1073/pnas.85.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. A., Murray N., Kennedy M., Koppula S. V., Tara D., Ansel J. C. Melanoma-derived interleukin 6 inhibits in vivo melanoma growth. J Invest Dermatol. 1994 Mar;102(3):278–284. doi: 10.1111/1523-1747.ep12371782. [DOI] [PubMed] [Google Scholar]
- Armstrong C. A., Tara D. C., Hart C. E., Köck A., Luger T. A., Ansel J. C. Heterogeneity of cytokine production by human malignant melanoma cells. Exp Dermatol. 1992 Jul;1(1):37–45. doi: 10.1111/j.1600-0625.1992.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Ballin M., Gomez D. E., Sinha C. C., Thorgeirsson U. P. Ras oncogene mediated induction of a 92 kDa metalloproteinase; strong correlation with the malignant phenotype. Biochem Biophys Res Commun. 1988 Aug 15;154(3):832–838. doi: 10.1016/0006-291x(88)90215-x. [DOI] [PubMed] [Google Scholar]
- Bennicelli J. L., Elias J., Kern J., Guerry D., 4th Production of interleukin 1 activity by cultured human melanoma cells. Cancer Res. 1989 Feb 15;49(4):930–935. [PubMed] [Google Scholar]
- Chen Q., Daniel V., Maher D. W., Hersey P. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int J Cancer. 1994 Mar 1;56(5):755–760. doi: 10.1002/ijc.2910560524. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Pigott D. A., Lazarus J. A. Ectopic peptides released by a human melanoma cell line that modulate the transformed phenotype. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5015–5019. doi: 10.1073/pnas.82.15.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone S., Marincola F. M. Loss of HLA class I antigens by melanoma cells: molecular mechanisms, functional significance and clinical relevance. Immunol Today. 1995 Oct;16(10):487–494. doi: 10.1016/0167-5699(95)80033-6. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Kwon B. S., Ghosh S., Delli Bovi P., Baird A. bFGF as an autocrine growth factor for human melanomas. Oncogene Res. 1988 Sep;3(2):177–186. [PubMed] [Google Scholar]
- Herlyn M. Human melanoma: development and progression. Cancer Metastasis Rev. 1990 Sep;9(2):101–112. doi: 10.1007/BF00046337. [DOI] [PubMed] [Google Scholar]
- Huang S., Luca M., Gutman M., McConkey D. J., Langley K. E., Lyman S. D., Bar-Eli M. Enforced c-KIT expression renders highly metastatic human melanoma cells susceptible to stem cell factor-induced apoptosis and inhibits their tumorigenic and metastatic potential. Oncogene. 1996 Dec 5;13(11):2339–2347. [PubMed] [Google Scholar]
- Huang S., Singh R. K., Xie K., Gutman M., Berry K. K., Bucana C. D., Fidler I. J., Bar-Eli M. Expression of the JE/MCP-1 gene suppresses metastatic potential in murine colon carcinoma cells. Cancer Immunol Immunother. 1994 Oct;39(4):231–238. doi: 10.1007/BF01525986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Xie K., Bucana C. D., Ullrich S. E., Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. 1996 Dec;2(12):1969–1979. [PubMed] [Google Scholar]
- Hudson J. M., Frade R., Bar-Eli M. Wild-type p53 regulates its own transcription in a cell-type specific manner. DNA Cell Biol. 1995 Sep;14(9):759–766. doi: 10.1089/dna.1995.14.759. [DOI] [PubMed] [Google Scholar]
- Huhtala P., Chow L. T., Tryggvason K. Structure of the human type IV collagenase gene. J Biol Chem. 1990 Jul 5;265(19):11077–11082. [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck A., Schwarz T., Urbanski A., Peng Z., Vetterlein M., Micksche M., Ansel J. C., Kung H. F., Luger T. A. Expression and release of interleukin-1 by different human melanoma cell lines. J Natl Cancer Inst. 1989 Jan 4;81(1):36–42. doi: 10.1093/jnci/81.1.36. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Riethmüller G., Johnson J. P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- Lu C., Kerbel R. S. Cytokines, growth factors and the loss of negative growth controls in the progression of human cutaneous malignant melanoma. Curr Opin Oncol. 1994 Mar;6(2):212–220. doi: 10.1097/00001622-199403000-00015. [DOI] [PubMed] [Google Scholar]
- Lu C., Vickers M. F., Kerbel R. S. Interleukin 6: a fibroblast-derived growth inhibitor of human melanoma cells from early but not advanced stages of tumor progression. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9215–9219. doi: 10.1073/pnas.89.19.9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca M., Hunt B., Bucana C. D., Johnson J. P., Fidler I. J., Bar-Eli M. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993 Feb;3(1):35–41. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Luca M., Xie S., Gutman M., Huang S., Bar-Eli M. Abnormalities in the CDKN2 (p16INK4/MTS-1) gene in human melanoma cells: relevance to tumor growth and metastasis. Oncogene. 1995 Oct 5;11(7):1399–1402. [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei S., Colombo M. P., Melani C., Silvani A., Parmiani G., Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994 Mar 15;56(6):853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- McGregor B. C., McGregor J. L., Weiss L. M., Wood G. S., Hu C. H., Boukerche H., Warnke R. A. Presence of cytoadhesins (IIb-IIIa-like glycoproteins) on human metastatic melanomas but not on benign melanocytes. Am J Clin Pathol. 1989 Oct;92(4):495–499. doi: 10.1093/ajcp/92.4.495. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Winkler A. B., Cavaliere R., Bigotti A., Ullrich A. Progression of human cutaneous melanoma is associated with loss of expression of c-kit proto-oncogene receptor. Int J Cancer. 1992 Sep 9;52(2):197–201. doi: 10.1002/ijc.2910520207. [DOI] [PubMed] [Google Scholar]
- Oxholm A., Oxholm P., Staberg B., Bendtzen K. Immunohistological detection of interleukin I-like molecules and tumour necrosis factor in human epidermis before and after UVB-irradiation in vivo. Br J Dermatol. 1988 Mar;118(3):369–376. doi: 10.1111/j.1365-2133.1988.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Radinsky R., Fidler I. J., Price J. E., Esumi N., Tsan R., Petty C. M., Bucana C. D., Bar-Eli M. Terminal differentiation and apoptosis in experimental lung metastases of human osteogenic sarcoma cells by wild type p53. Oncogene. 1994 Jul;9(7):1877–1883. [PubMed] [Google Scholar]
- Radinsky R. Growth factors and their receptors in metastasis. Semin Cancer Biol. 1991 Jun;2(3):169–177. [PubMed] [Google Scholar]
- Ray J. M., Stetler-Stevenson W. G. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995 Mar 1;14(5):908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich R., Blumenthal M., Liscovitch M. Role of phospholipase D in laminin-induced production of gelatinase A (MMP-2) in metastatic cells. Clin Exp Metastasis. 1995 Mar;13(2):134–140. doi: 10.1007/BF00133618. [DOI] [PubMed] [Google Scholar]
- Reich R., Thompson E. W., Iwamoto Y., Martin G. R., Deason J. R., Fuller G. C., Miskin R. Effects of inhibitors of plasminogen activator, serine proteinases, and collagenase IV on the invasion of basement membranes by metastatic cells. Cancer Res. 1988 Jun 15;48(12):3307–3312. [PubMed] [Google Scholar]
- Richmond A., Lawson D. H., Nixon D. W., Stevens J. S., Chawla R. K. Extraction of a melanoma growth-stimulatory activity from culture medium conditioned by the Hs0294 human melanoma cell line. Cancer Res. 1983 May;43(5):2106–2112. [PubMed] [Google Scholar]
- Schadendorf D., Fichtner I., Makki A., Alijagic S., Küpper M., Mrowietz U., Henz B. M. Metastatic potential of human melanoma cells in nude mice--characterisation of phenotype, cytokine secretion and tumour-associated antigens. Br J Cancer. 1996 Jul;74(2):194–199. doi: 10.1038/bjc.1996.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., Möller A., Algermissen B., Worm M., Sticherling M., Czarnetzki B. M. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993 Sep 1;151(5):2667–2675. [PubMed] [Google Scholar]
- Scheibenbogen C., Möhler T., Haefele J., Hunstein W., Keilholz U. Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load. Melanoma Res. 1995 Jun;5(3):179–181. doi: 10.1097/00008390-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Schönbeck U., Brandt E., Petersen F., Flad H. D., Loppnow H. IL-8 specifically binds to endothelial but not to smooth muscle cells. J Immunol. 1995 Mar 1;154(5):2375–2383. [PubMed] [Google Scholar]
- Singh R. K., Gutman M., Llansa N., Fidler I. J. Interferon-beta prevents the upregulation of interleukin-8 expression in human melanoma cells. J Interferon Cytokine Res. 1996 Aug;16(8):577–584. doi: 10.1089/jir.1996.16.577. [DOI] [PubMed] [Google Scholar]
- Singh R. K., Gutman M., Radinsky R., Bucana C. D., Fidler I. J. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res. 1994 Jun 15;54(12):3242–3247. [PubMed] [Google Scholar]
- Singh R. K., Gutman M., Reich R., Bar-Eli M. Ultraviolet B irradiation promotes tumorigenic and metastatic properties in primary cutaneous melanoma via induction of interleukin 8. Cancer Res. 1995 Aug 15;55(16):3669–3674. [PubMed] [Google Scholar]
- Sozzani S., Agwu D. E., Ellenburg M. D., Locati M., Rieppi M., Rojas A., Mantovani A., McPhail L. C. Activation of phospholipase D by interleukin-8 in human neutrophils. Blood. 1994 Dec 1;84(11):3895–3901. [PubMed] [Google Scholar]
- Sticherling M., Hetzel F., Schröder J. M., Christophers E. Time- and stimulus-dependent secretion of NAP-1/IL-8 by human fibroblasts and endothelial cells. J Invest Dermatol. 1993 Oct;101(4):573–576. doi: 10.1111/1523-1747.ep12366023. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Elner V. M., Martonyi C. L., Koch A. E., Polverini P. J., Elner S. G. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992 Dec;141(6):1279–1284. [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson U. P., Turpeenniemi-Hujanen T., Williams J. E., Westin E. H., Heilman C. A., Talmadge J. E., Liotta L. A. NIH/3T3 cells transfected with human tumor DNA containing activated ras oncogenes express the metastatic phenotype in nude mice. Mol Cell Biol. 1985 Jan;5(1):259–262. doi: 10.1128/mcb.5.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschraegen C. F., Giovanella B. C., Mendoza J. T., Kozielski A. J., Stehlin J. S., Jr Specific organ metastases of human melanoma cells injected into the arterial circulation of nude mice. Anticancer Res. 1991 Mar-Apr;11(2):529–535. [PubMed] [Google Scholar]
- Wang J. M., Taraboletti G., Matsushima K., Van Damme J., Mantovani A. Induction of haptotactic migration of melanoma cells by neutrophil activating protein/interleukin-8. Biochem Biophys Res Commun. 1990 May 31;169(1):165–170. doi: 10.1016/0006-291x(90)91449-3. [DOI] [PubMed] [Google Scholar]
- Westermark B., Johnsson A., Paulsson Y., Betsholtz C., Heldin C. H., Herlyn M., Rodeck U., Koprowski H. Human melanoma cell lines of primary and metastatic origin express the genes encoding the chains of platelet-derived growth factor (PDGF) and produce a PDGF-like growth factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7197–7200. doi: 10.1073/pnas.83.19.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut R., Perlis R., Eliyahu S., Yarden Y., Givol D., Lyman S. D., Halaban R. KIT ligand (mast cell growth factor) inhibits the growth of KIT-expressing melanoma cells. Oncogene. 1993 Aug;8(8):2221–2229. [PubMed] [Google Scholar]