Abstract
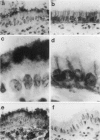
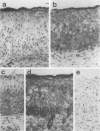
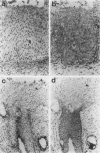
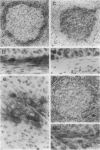
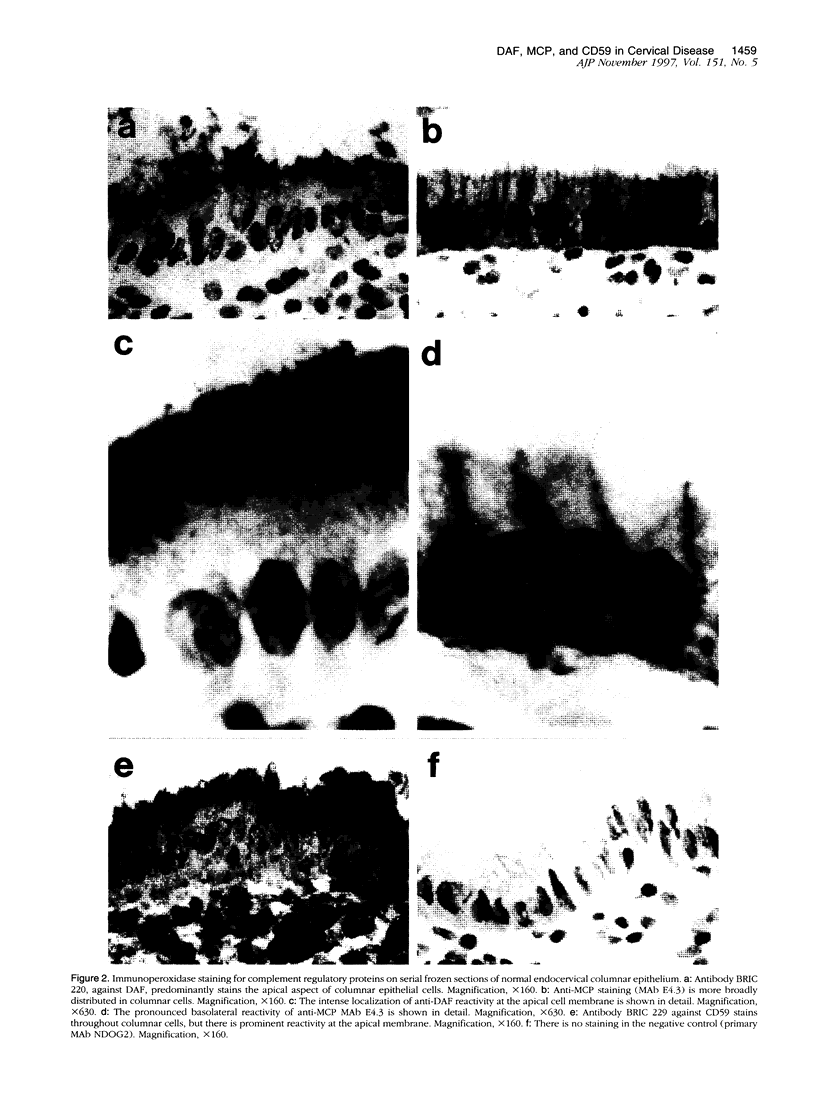
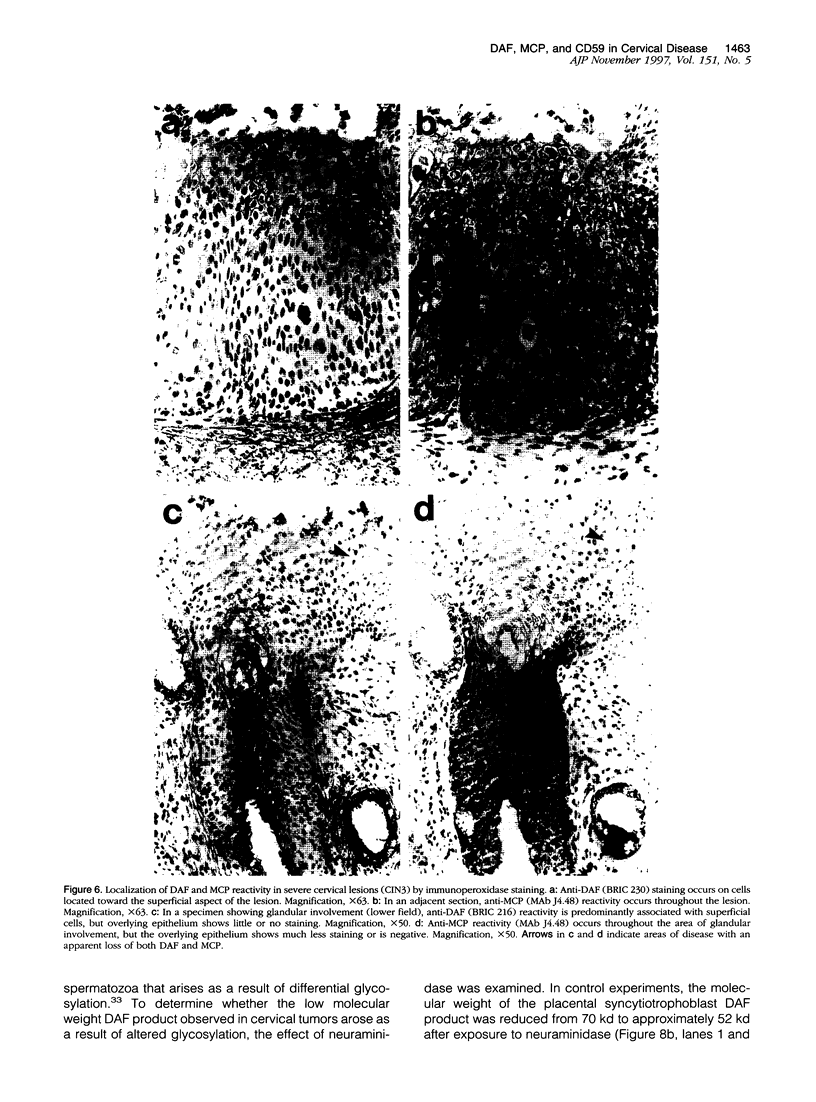
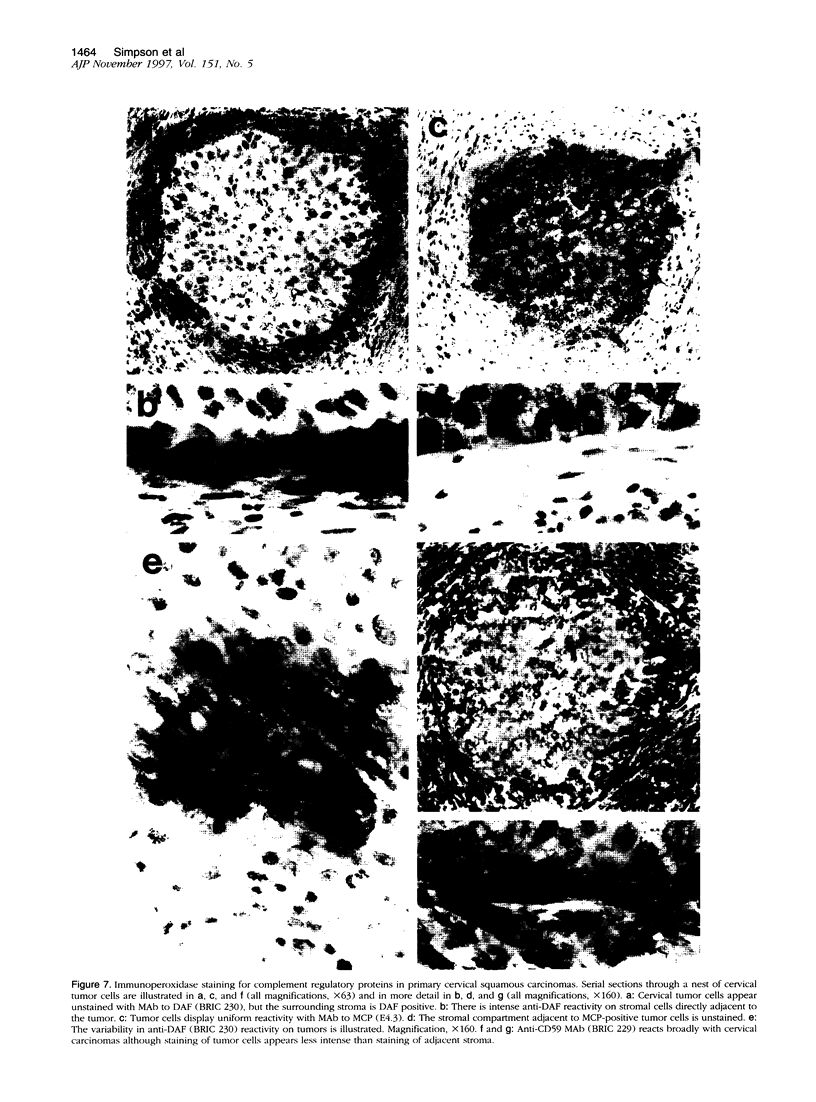
The membrane-bound complement regulators decay-accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46), and CD59 are broadly expressed proteins that act together to protect host tissues from autologous complement. Comparison of expression profiles of these proteins between normal and pathological tissues could reveal a mechanism by which tumor cells evade complement-mediated killing. Expression of the regulators was therefore examined in the normal human uterine cervix, in cervical intraepithelial neoplasia (CIN; n = 23), and in cervical squamous carcinomas (n = 6). DAF and MCP were reciprocally expressed in normal ectocervical epithelium. MCP was confined predominantly to the basal and parabasal layers with more extensive expression in metaplastic squamous epithelium. An apparent expansion in MCP expression was observed in more severe premalignant lesions whereas cervical carcinoma were uniformly MCP positive. By contrast, DAF expression appeared unaltered in premalignant lesions and variable in carcinomas. However, increased DAF was observed in stromal cells directly adjacent to infiltrating tumor cells. A low molecular weight DAF product was detected in tumors, and preliminary evidence suggests this may be derived from stromal cells. Overall, changes in expression of C3 convertase regulators in both the stromal and epithelial compartments may be important for evasion of immune surveillance in cervical cancer.
Full text
PDF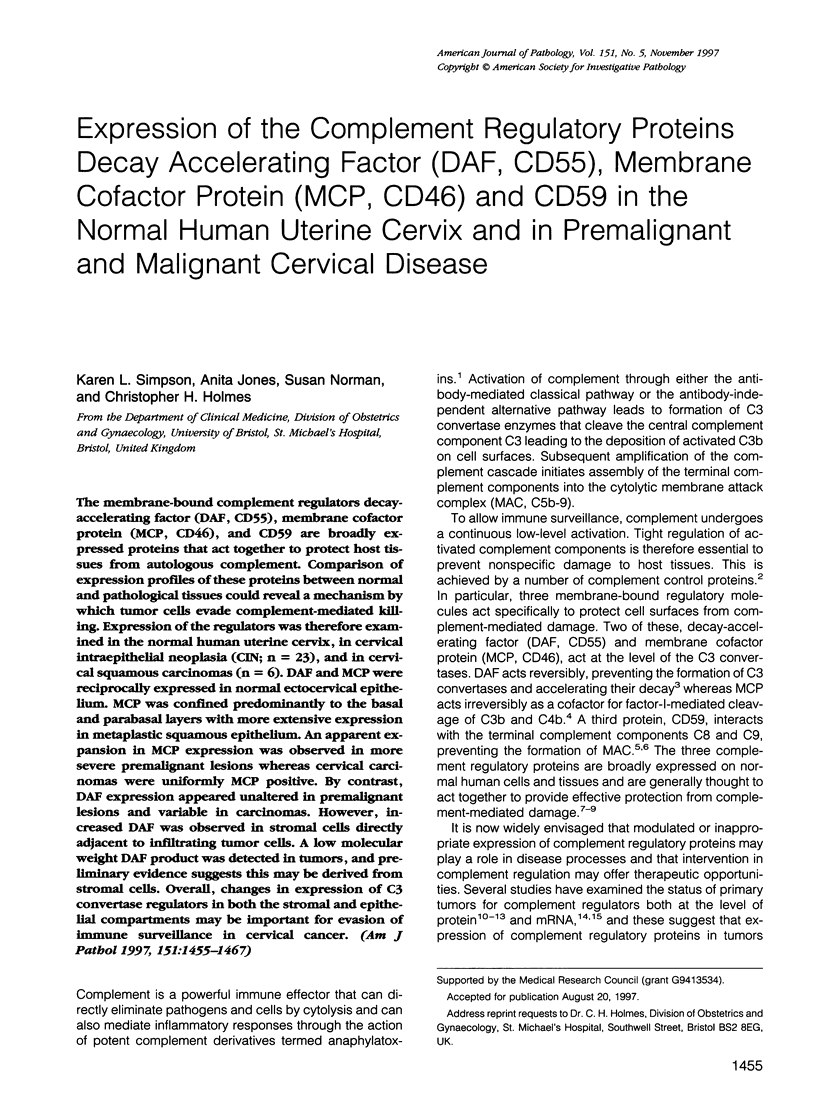








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergelson J. M., Chan M., Solomon K. R., St John N. F., Lin H., Finberg R. W. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6245–6248. doi: 10.1073/pnas.91.13.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørge L., Jensen T. S., Matre R. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol Immunother. 1996 Mar;42(3):185–192. doi: 10.1007/s002620050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørge L., Jensen T. S., Ulvestad E., Vedeler C. A., Matre R. The influence of tumour necrosis factor-alpha, interleukin-1 beta and interferon-gamma on the expression and function of the complement regulatory protein CD59 on the human colonic adenocarcinoma cell line HT29. Scand J Immunol. 1995 Apr;41(4):350–356. doi: 10.1111/j.1365-3083.1995.tb03578.x. [DOI] [PubMed] [Google Scholar]
- Bora N. S., Post T. W., Atkinson J. P. Membrane cofactor protein of the complement system. A HindIII restriction fragment length polymorphism that correlates with the expression polymorphism. J Immunol. 1991 Apr 15;146(8):2821–2825. [PubMed] [Google Scholar]
- Bryant R. W., Granzow C. A., Siegel M. I., Egan R. W., Billah M. M. Wheat germ agglutinin and other selected lectins increase synthesis of decay-accelerating factor in human endothelial cells. J Immunol. 1991 Sep 15;147(6):1856–1862. [PubMed] [Google Scholar]
- Bryant R. W., Granzow C. A., Siegel M. I., Egan R. W., Billah M. M. Wheat germ agglutinin and other selected lectins increase synthesis of decay-accelerating factor in human endothelial cells. J Immunol. 1991 Sep 15;147(6):1856–1862. [PubMed] [Google Scholar]
- Cervoni F., Oglesby T. J., Fénichel P., Dohr G., Rossi B., Atkinson J. P., Hsi B. L. Expression of decay-accelerating factor (CD55) of the complement system on human spermatozoa. J Immunol. 1993 Jul 15;151(2):939–948. [PubMed] [Google Scholar]
- Cheung N. K., Walter E. I., Smith-Mensah W. H., Ratnoff W. D., Tykocinski M. L., Medof M. E. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988 Apr;81(4):1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinek T., Horejsí V. The nature of large noncovalent complexes containing glycosyl-phosphatidylinositol-anchored membrane glycoproteins and protein tyrosine kinases. J Immunol. 1992 Oct 1;149(7):2262–2270. [PubMed] [Google Scholar]
- Davis L. S., Patel S. S., Atkinson J. P., Lipsky P. E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988 Oct 1;141(7):2246–2252. [PubMed] [Google Scholar]
- Dörig R. E., Marcil A., Chopra A., Richardson C. D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell. 1993 Oct 22;75(2):295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Fletcher A., Bryant J. A., Gardner B., Judson P. A., Spring F. A., Parsons S. F., Mallinson G., Anstee D. J. New monoclonal antibodies in CD59: use for the analysis of peripheral blood cells from paroxysmal nocturnal haemoglobinuria (PNH) patients and for the quantitation of CD59 on normal and decay accelerating factor (DAF)-deficient erythrocytes. Immunology. 1992 Mar;75(3):507–512. [PMC free article] [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Okada H., Okada N., Wainwright S. D., Purcell D. F., Houlihan J. M. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55). Eur J Immunol. 1992 Jun;22(6):1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Wainwright S. D., Tate C. G., Houlihan J. M., Sawyer I. H., Rogers I. P., Spring F. A., Anstee D. J., Tanner M. J. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990 Apr 15;144(8):3099–3105. [PubMed] [Google Scholar]
- Jensen T. S., Bjørge L., Wollen A. L., Ulstein M. Identification of the complement regulatory proteins CD46, CD55, and CD59 in human fallopian tube, endometrium, and cervical mucosa and secretion. Am J Reprod Immunol. 1995 Jul;34(1):1–9. doi: 10.1111/j.1600-0897.1995.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita T. Biology of complement: the overture. Immunol Today. 1991 Sep;12(9):291–295. doi: 10.1016/0167-5699(91)90001-A. [DOI] [PubMed] [Google Scholar]
- Korty P. E., Brando C., Shevach E. M. CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol. 1991 Jun 15;146(12):4092–4098. [PubMed] [Google Scholar]
- Kumar S., Vinci J. M., Pytel B. A., Baglioni C. Expression of messenger RNAs for complement inhibitors in human tissues and tumors. Cancer Res. 1993 Jan 15;53(2):348–353. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Tedja I., Atkinson J. P. Membrane cofactor protein (CD46) of complement. Processing differences related to alternatively spliced cytoplasmic domains. J Biol Chem. 1994 Apr 8;269(14):10776–10779. [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- McNearney T., Ballard L., Seya T., Atkinson J. P. Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest. 1989 Aug;84(2):538–545. doi: 10.1172/JCI114196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S., Morgan B. P., Davies A., Daniels R. H., Olavesen M. G., Waldmann H., Lachmann P. J. Human protectin (CD59), an 18,000-20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology. 1990 Sep;71(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Naniche D., Varior-Krishnan G., Cervoni F., Wild T. F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993 Oct;67(10):6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehans G. A., Cherwitz D. L., Staley N. A., Knapp D. J., Dalmasso A. P. Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol. 1996 Jul;149(1):129–142. [PMC free article] [PubMed] [Google Scholar]
- Nose M., Katoh M., Okada N., Kyogoku M., Okada H. Tissue distribution of HRF20, a novel factor preventing the membrane attack of homologous complement, and its predominant expression on endothelial cells in vivo. Immunology. 1990 Jun;70(2):145–149. [PMC free article] [PubMed] [Google Scholar]
- Nowicki B., Hart A., Coyne K. E., Lublin D. M., Nowicki S. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J Exp Med. 1993 Dec 1;178(6):2115–2121. doi: 10.1084/jem.178.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oglesby T. J., Longwith J. E., Huettner P. C. Human complement regulator expression by the normal female reproductive tract. Anat Rec. 1996 Sep;246(1):78–86. doi: 10.1002/(SICI)1097-0185(199609)246:1<78::AID-AR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Okada N., Liszewski M. K., Atkinson J. P., Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2489–2493. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesando J. M., Stucki M., Hoffman P. Altered expression of surface antigens with appearance of HLA class II molecules on a malignant human B-cell line. Hum Immunol. 1987 Aug;19(4):235–243. doi: 10.1016/0198-8859(87)90041-3. [DOI] [PubMed] [Google Scholar]
- Price R. J., Boettcher B. The presence of complement in human cervical mucus and its possible relevance to infertility in women with complement-dependent sperm-immobilizing antibodies. Fertil Steril. 1979 Jul;32(1):61–66. doi: 10.1016/s0015-0282(16)44117-8. [DOI] [PubMed] [Google Scholar]
- Rollins S. A., Zhao J., Ninomiya H., Sims P. J. Inhibition of homologous complement by CD59 is mediated by a species-selective recognition conferred through binding to C8 within C5b-8 or C9 within C5b-9. J Immunol. 1991 Apr 1;146(7):2345–2351. [PubMed] [Google Scholar]
- Russell S. M., Sparrow R. L., McKenzie I. F., Purcell D. F. Tissue-specific and allelic expression of the complement regulator CD46 is controlled by alternative splicing. Eur J Immunol. 1992 Jun;22(6):1513–1518. doi: 10.1002/eji.1830220625. [DOI] [PubMed] [Google Scholar]
- Sayama K., Shiraishi S., Miki Y. Distribution of complement regulators (CD46, CD55 and CD59) in skin appendages, and in benign and malignant skin neoplasms. Br J Dermatol. 1992 Jul;127(1):1–4. doi: 10.1111/j.1365-2133.1992.tb14814.x. [DOI] [PubMed] [Google Scholar]
- Sayama K., Shiraishi S., Shirakata Y., Kobayashi Y., Miki Y. Characterization of decay-accelerating factor (DAF) in human skin. J Invest Dermatol. 1991 Jan;96(1):61–64. doi: 10.1111/1523-1747.ep12514737. [DOI] [PubMed] [Google Scholar]
- Seya T., Atkinson J. P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989 Dec 1;264(2):581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seya T., Hara T., Matsumoto M., Akedo H. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol. 1990 Jul 1;145(1):238–245. [PubMed] [Google Scholar]
- Shibata T., Cosio F. G., Birmingham D. J. Complement activation induces the expression of decay-accelerating factor on human mesangial cells. J Immunol. 1991 Dec 1;147(11):3901–3908. [PubMed] [Google Scholar]
- Shinoura N., Heffelfinger S. C., Miller M., Shamraj O. I., Miura N. H., Larson J. J., DeTribolet N., Warnick R. E., Tew J. J., Menon A. G. RNA expression of complement regulatory proteins in human brain tumors. Cancer Lett. 1994 Nov 11;86(2):143–149. doi: 10.1016/0304-3835(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Simpson K. L., Holmes C. H. Differential expression of complement regulatory proteins decay-accelerating factor (CD55), membrane cofactor protein (CD46) and CD59 during human spermatogenesis. Immunology. 1994 Mar;81(3):452–461. [PMC free article] [PubMed] [Google Scholar]
- Simpson K. L., Houlihan J. M., Holmes C. H. Complement regulatory proteins in early human fetal life: CD59, membrane co-factor protein (MCP) and decay-accelerating factor (DAF) are differentially expressed in the developing liver. Immunology. 1993 Oct;80(2):183–190. [PMC free article] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Sparrow R. L., McKenzie I. F. Hu Ly-m5: a unique antigen physically associated with HLA molecules. Hum Immunol. 1983 May;7(1):1–15. doi: 10.1016/0198-8859(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., Davies J. O., Stirrat G. M. Immunohistology of normal and ovarian cancer tissue with a monoclonal antibody to placental alkaline phosphatase. Cancer Res. 1984 Oct;44(10):4496–4502. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji S., Kaji K., Nagasawa S. Decay-accelerating factor on human umbilical vein endothelial cells. Its histamine-induced expression and spontaneous rapid shedding from the cell surface. J Immunol. 1994 Feb 1;152(3):1404–1410. [PubMed] [Google Scholar]
- Yamakawa M., Yamada K., Tsuge T., Ohrui H., Ogata T., Dobashi M., Imai Y. Protection of thyroid cancer cells by complement-regulatory factors. Cancer. 1994 Jun 1;73(11):2808–2817. doi: 10.1002/1097-0142(19940601)73:11<2808::aid-cncr2820731125>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]