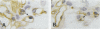Abstract
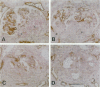
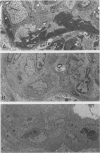
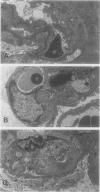
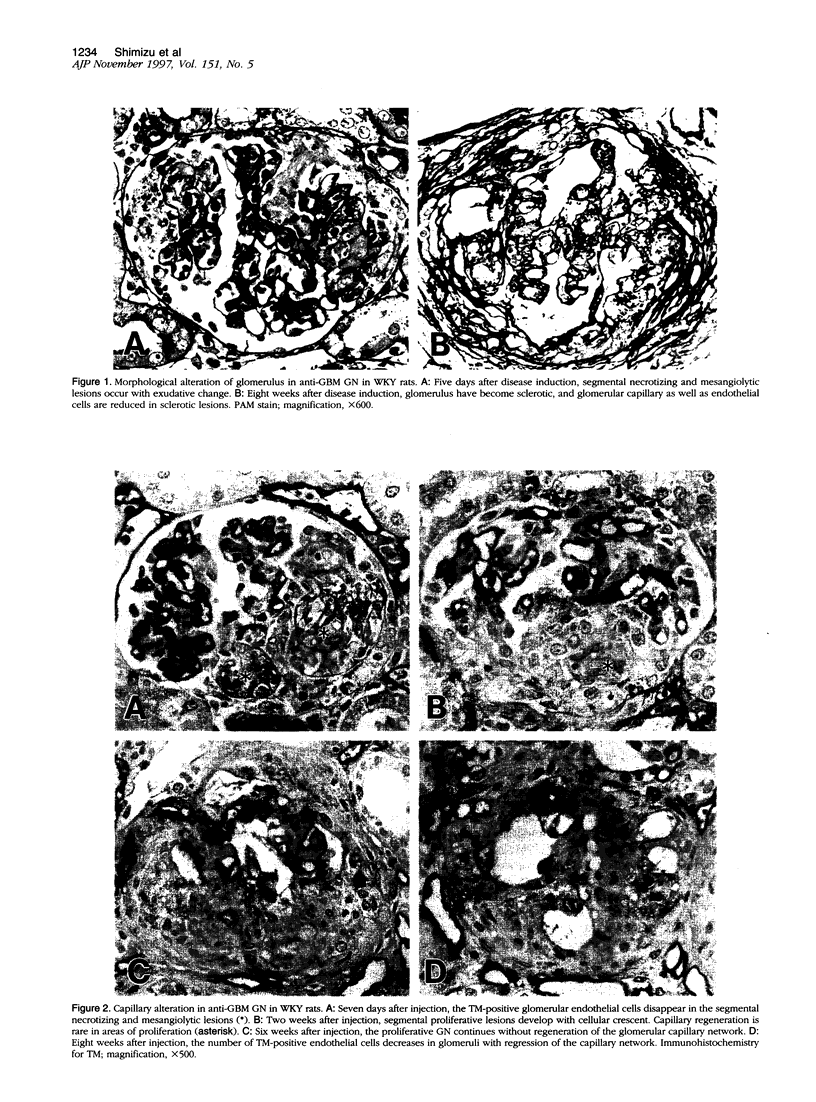
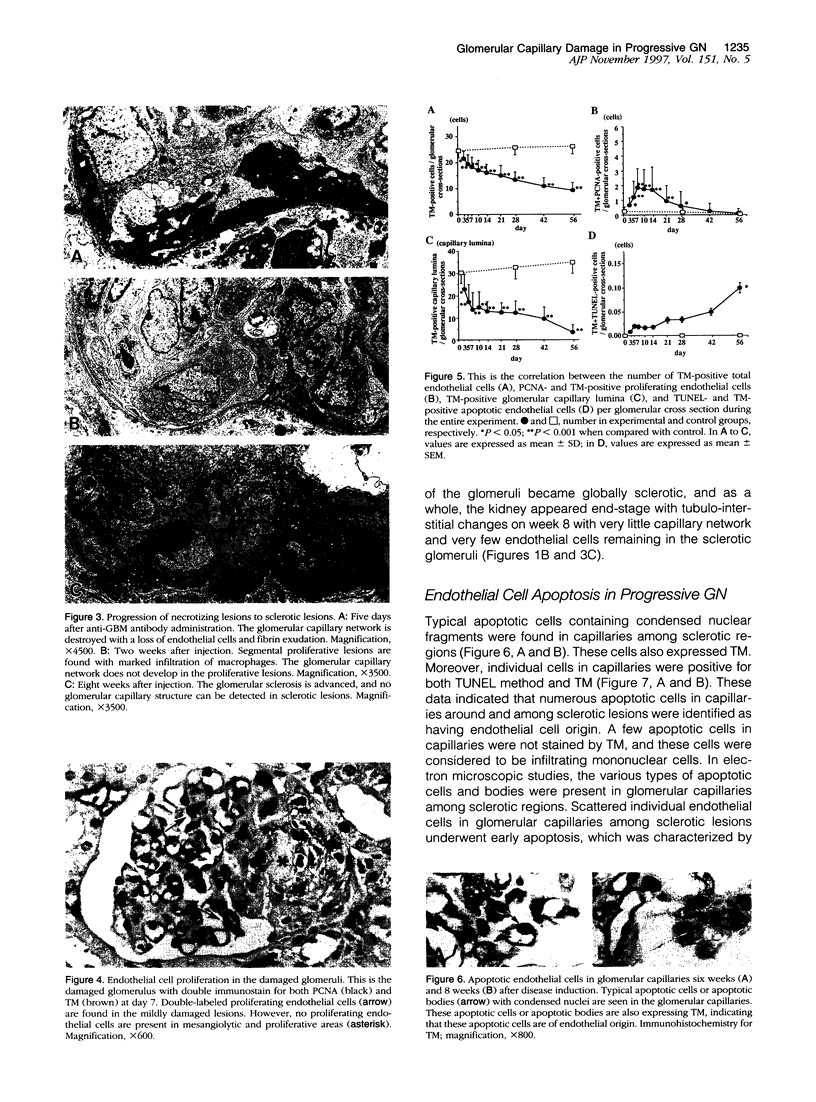
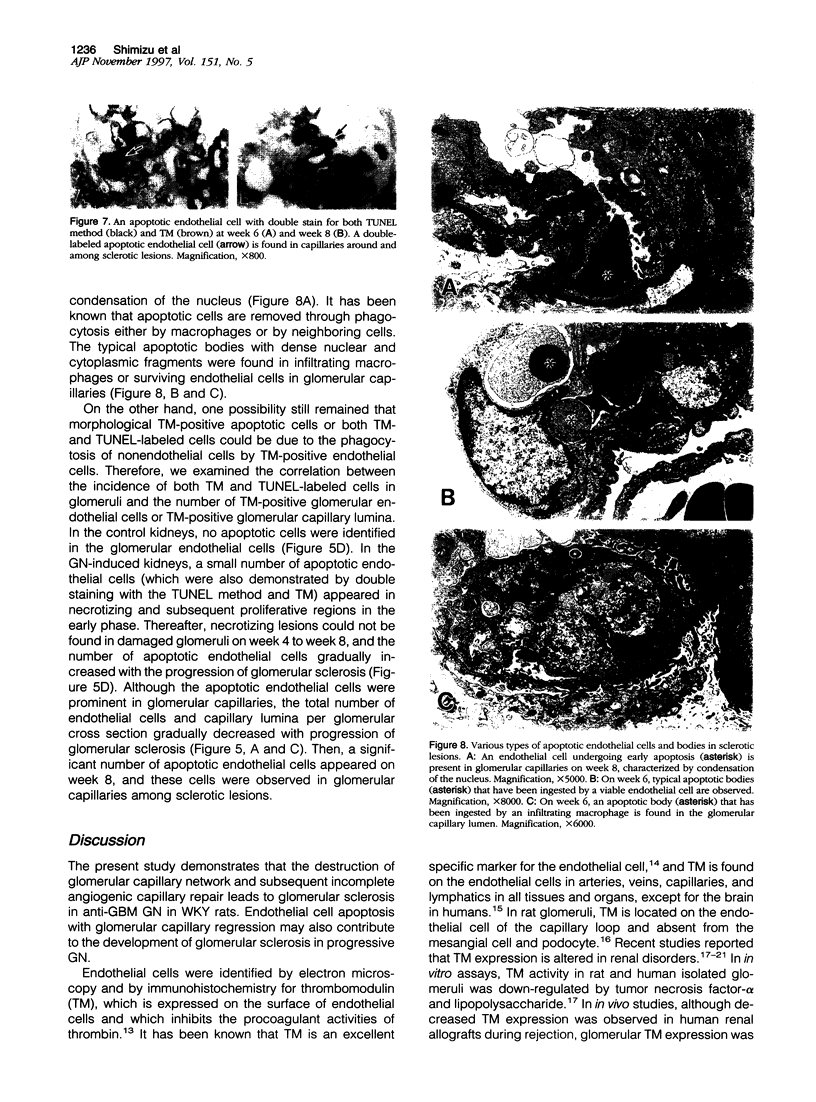
Glomerulonephritis (GN) leading to glomerular sclerosis remains an important cause of renal failure. The glomerulus is a capillary network, but endothelial and vascular reactions during progressive GN are not well understood. We have, therefore, examined the morphological alterations of glomerular capillary network and endothelial cells during the progression of damaged glomeruli to glomerular sclerosis. A progressive model of anti-glomerular basement membrane (GBM) GN was induced in Wistar-Kyoto (WKY) rats with a single injection of anti-rat GBM antibody. Severe necrotizing glomerular injuries were observed between day 5 and week 3 with a reduction in the number of total glomerular endothelial cells and total glomerular capillary lumina per glomerular cross sections. In necrotizing lesions, the glomerular endothelial cells were lost with the destruction of the glomerular capillary network. Moreover, angiogenic capillary repair with proliferation of endothelial cells was rare in severely damaged regions of glomeruli. Subsequently, mesangial hypercellularity and marked mesangial matrix accumulation occurred with absence of the development of a capillary network, and the necrotizing lesions progressed to sclerotic scars until 8 weeks. Although active necrotizing lesions could not be seen in damaged glomeruli between week 4 and week 8, the number of apoptotic endothelial cells gradually increased in the glomerular capillaries (0.10 +/- 0.01 apoptotic endothelial cells/glomerular cross section at week 8 versus 0.00 +/- 0.00 control cells (mean +/- SEM; P < 0.05) with the progression of glomerular sclerosis. Whereas the number of apoptotic endothelial cells increased in the damaged glomeruli, the number of total glomerular endothelial cells decreased (9.3 +/- 3.0 cells/glomerular cross section at week 8 versus 24.8 +/- 3.0 cells in control (mean +/- SD); P < 0.001) with regression of glomerular capillaries (3.6 +/- 2.5 capillary lumina/glomerular cross section at week 8 versus 35.0 +/- 5.0 capillary lumina in control (mean +/- SD); P < 0.001). Finally, glomerular endothelial cells could not be detected in the sclerotic lesions in progressive anti-GBM GN in WKY rats. These data indicate that the destruction of the capillary network of glomeruli and subsequent incomplete angiogenic capillary repair leads to glomerular sclerosis in progressive GN. Endothelial cell apoptosis with glomerular capillary regression may also contribute to the development of glomerular sclerosis. Injury of the glomerular capillary network with endothelial cell damage, including apoptosis and subsequent incomplete capillary repair, plays an important role in the progression of glomerular sclerosis during anti-GBM GN in WKY rats.
Full text
PDF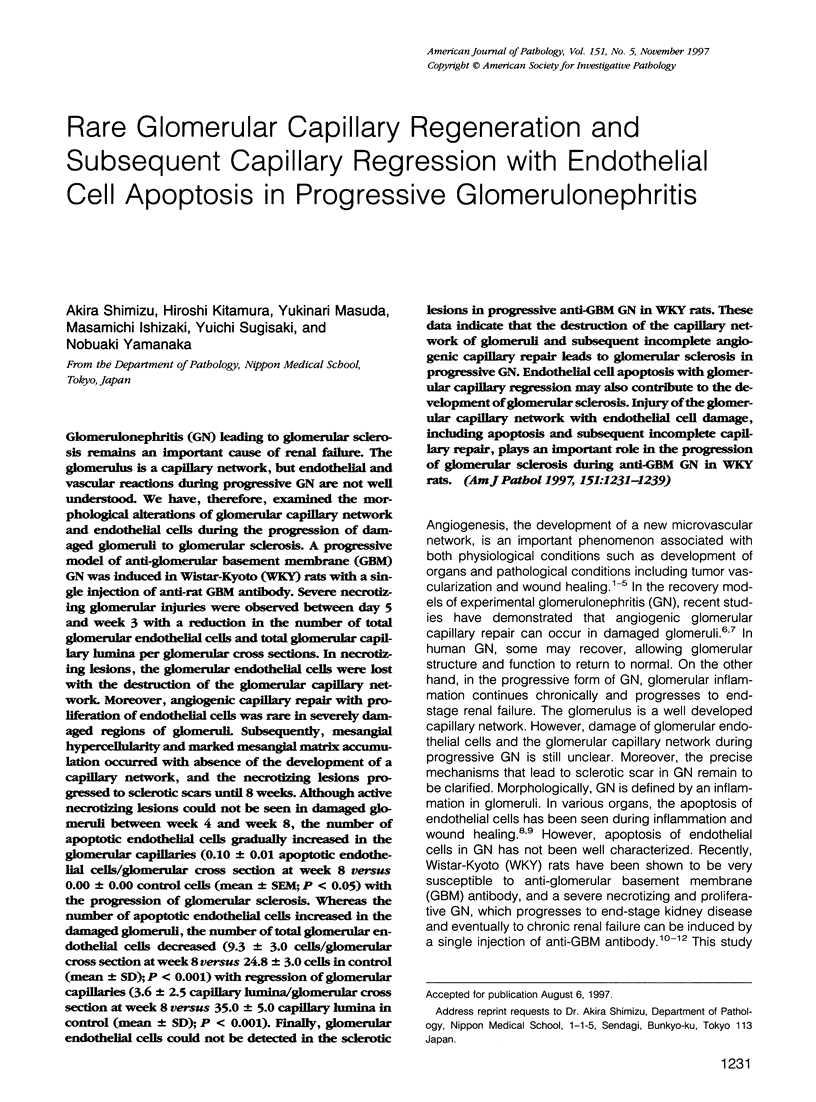





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki S., Ishida T., Yamamoto T., Kaji K., Hayashi H. Induction of apoptosis by hemorrhagic snake venom in vascular endothelial cells. Biochem Biophys Res Commun. 1993 Jan 15;190(1):148–153. doi: 10.1006/bbrc.1993.1023. [DOI] [PubMed] [Google Scholar]
- Araki S., Shimada Y., Kaji K., Hayashi H. Apoptosis of vascular endothelial cells by fibroblast growth factor deprivation. Biochem Biophys Res Commun. 1990 May 16;168(3):1194–1200. doi: 10.1016/0006-291x(90)91155-l. [DOI] [PubMed] [Google Scholar]
- Araki S., Simada Y., Kaji K., Hayashi H. Role of protein kinase C in the inhibition by fibroblast growth factor of apoptosis in serum-depleted endothelial cells. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1081–1085. doi: 10.1016/0006-291x(90)91557-9. [DOI] [PubMed] [Google Scholar]
- Arnold F., West D. C. Angiogenesis in wound healing. Pharmacol Ther. 1991 Dec;52(3):407–422. doi: 10.1016/0163-7258(91)90034-j. [DOI] [PubMed] [Google Scholar]
- Azmi T. I., O'Shea J. D. Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab Invest. 1984 Aug;51(2):206–217. [PubMed] [Google Scholar]
- Berke G. Unlocking the secrets of CTL and NK cells. Immunol Today. 1995 Jul;16(7):343–346. doi: 10.1016/0167-5699(95)80152-9. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Esmon N. L., Olson J. R., Esmon C. T. Distribution of the thrombomodulin antigen in the rabbit vasculature. Lab Invest. 1986 Feb;54(2):172–178. [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Karnovsky M. J. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988 May;33(5):917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- Duijvestijn A. M., van Goor H., Klatter F., Majoor G. D., van Bussel E., van Breda Vriesman P. J. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992 Apr;66(4):459–466. [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995 Dec 28;333(26):1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. J., Kanfer A. Quantification and modulation of thrombomodulin activity in isolated rat and human glomeruli. Kidney Int. 1992 May;41(5):1170–1174. doi: 10.1038/ki.1992.178. [DOI] [PubMed] [Google Scholar]
- Horvat R., Palade G. E. Thrombomodulin and thrombin localization on the vascular endothelium; their internalization and transcytosis by plasmalemmal vesicles. Eur J Cell Biol. 1993 Aug;61(2):299–313. [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hyink D. P., Abrahamson D. R. Origin of the glomerular vasculature in the developing kidney. Semin Nephrol. 1995 Jul;15(4):300–314. [PubMed] [Google Scholar]
- Iruela-Arispe L., Gordon K., Hugo C., Duijvestijn A. M., Claffey K. P., Reilly M., Couser W. G., Alpers C. E., Johnson R. J. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am J Pathol. 1995 Dec;147(6):1715–1727. [PMC free article] [PubMed] [Google Scholar]
- JONES D. B. Inflammation and repair of glomerulus. Am J Pathol. 1951 Nov-Dec;27(6):991–1009. [PMC free article] [PubMed] [Google Scholar]
- Kawakami A., Tian Q., Duan X., Streuli M., Schlossman S. F., Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K., Yaoita E., Yamamoto T., Kihara I. Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int. 1992 Jun;41(6):1517–1526. doi: 10.1038/ki.1992.221. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Yaoita E., Yamamoto T., Tamatani T., Miyasaka M., Kihara I. Antibodies against intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 prevent glomerular injury in rat experimental crescentic glomerulonephritis. J Immunol. 1993 Feb 1;150(3):1074–1083. [PubMed] [Google Scholar]
- Kitamura H., Sugisaki Y., Yamanaka N. Endothelial regeneration during the repair process following Habu-snake venom induced glomerular injury. Virchows Arch. 1995;427(2):195–204. doi: 10.1007/BF00196526. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., D'Amore P. A. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Kobayashi Y. Cellular aspects of rabbit Masugi nephritis. II. Progressive glomerular injuries with crescent formation. Lab Invest. 1972 Dec;27(6):620–631. [PubMed] [Google Scholar]
- Lan H. Y., Mu W., Nikolic-Paterson D. J., Atkins R. C. A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens. J Histochem Cytochem. 1995 Jan;43(1):97–102. doi: 10.1177/43.1.7822770. [DOI] [PubMed] [Google Scholar]
- Laszik Z., Carson C. W., Nadasdy T., Johnson L. D., Lerner M. R., Brackett D. J., Esmon C. T., Silva F. G. Lack of suppressed renal thrombomodulin expression in a septic rat model with glomerular thrombotic microangiopathy. Lab Invest. 1994 Jun;70(6):862–867. [PubMed] [Google Scholar]
- Latker C. H., Feinberg R. N., Beebe D. C. Localized vascular regression during limb morphogenesis in the chicken embryo: II. Morphological changes in the vasculature. Anat Rec. 1986 Apr;214(4):410-7, 392-3. doi: 10.1002/ar.1092140412. [DOI] [PubMed] [Google Scholar]
- Lizard G., Deckert V., Dubrez L., Moisant M., Gambert P., Lagrost L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am J Pathol. 1996 May;148(5):1625–1638. [PMC free article] [PubMed] [Google Scholar]
- Maruyama I., Bell C. E., Majerus P. W. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985 Aug;101(2):363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M., Yuzawa Y., Maruyama I., Sakamoto N., Matsuo S. Glomerular localization of thrombomodulin in human glomerulonephritis. Lab Invest. 1993 Aug;69(2):193–202. [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy M. A., Schwartz S. M. A technique to investigate surface morphology and endothelial cell replication of small arteries: a study in acute angiotensin-induced hypertensive rats. Microvasc Res. 1982 Sep;24(2):158–167. doi: 10.1016/0026-2862(82)90053-x. [DOI] [PubMed] [Google Scholar]
- Robaye B., Mosselmans R., Fiers W., Dumont J. E., Galand P. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol. 1991 Feb;138(2):447–453. [PMC free article] [PubMed] [Google Scholar]
- Roux F., Durieu-Trautmann O., Chaverot N., Claire M., Mailly P., Bourre J. M., Strosberg A. D., Couraud P. O. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994 Apr;159(1):101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Savill J. Apoptosis and the kidney. J Am Soc Nephrol. 1994 Jul;5(1):12–21. doi: 10.1681/ASN.V5112. [DOI] [PubMed] [Google Scholar]
- Savill J., Johnson R. J. Glomerular remodelling after inflammatory injury. Exp Nephrol. 1995 May-Jun;3(3):149–158. [PubMed] [Google Scholar]
- Shigematsu H., Kobayashi Y. The distortion and disorganization of the glomerulus in progressive Masugi nephritis in the rat. Virchows Arch B Cell Pathol. 1973 Dec 17;14(4):313–328. doi: 10.1007/BF02889197. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Masuda Y., Kitamura H., Ishizaki M., Sugisaki Y., Yamanaka N. Apoptosis in progressive crescentic glomerulonephritis. Lab Invest. 1996 May;74(5):941–951. [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995 Mar 10;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Tomura S., Deguchi F., Marumo F., Aoki N. Enhanced presence of thrombomodulin in the glomeruli of lupus glomerulonephritis. Clin Nephrol. 1994 Apr;41(4):205–210. [PubMed] [Google Scholar]
- Tsuchida A., Salem H., Thomson N., Hancock W. W. Tumor necrosis factor production during human renal allograft rejection is associated with depression of plasma protein C and free protein S levels and decreased intragraft thrombomodulin expression. J Exp Med. 1992 Jan 1;175(1):81–90. doi: 10.1084/jem.175.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L. Toward an understanding of the molecular mechanisms of physiological cell death. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):786–789. doi: 10.1073/pnas.90.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- Yang J. J., Kettritz R., Falk R. J., Jennette J. C., Gaido M. L. Apoptosis of endothelial cells induced by the neutrophil serine proteases proteinase 3 and elastase. Am J Pathol. 1996 Nov;149(5):1617–1626. [PMC free article] [PubMed] [Google Scholar]