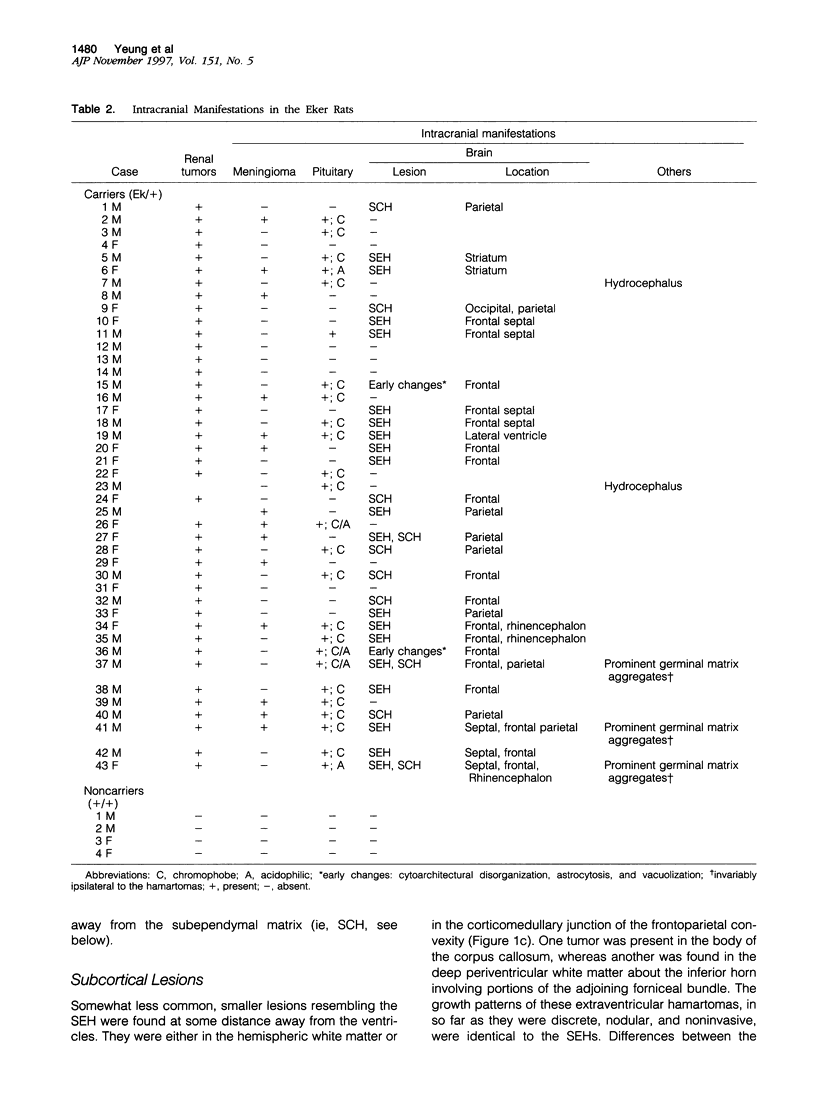
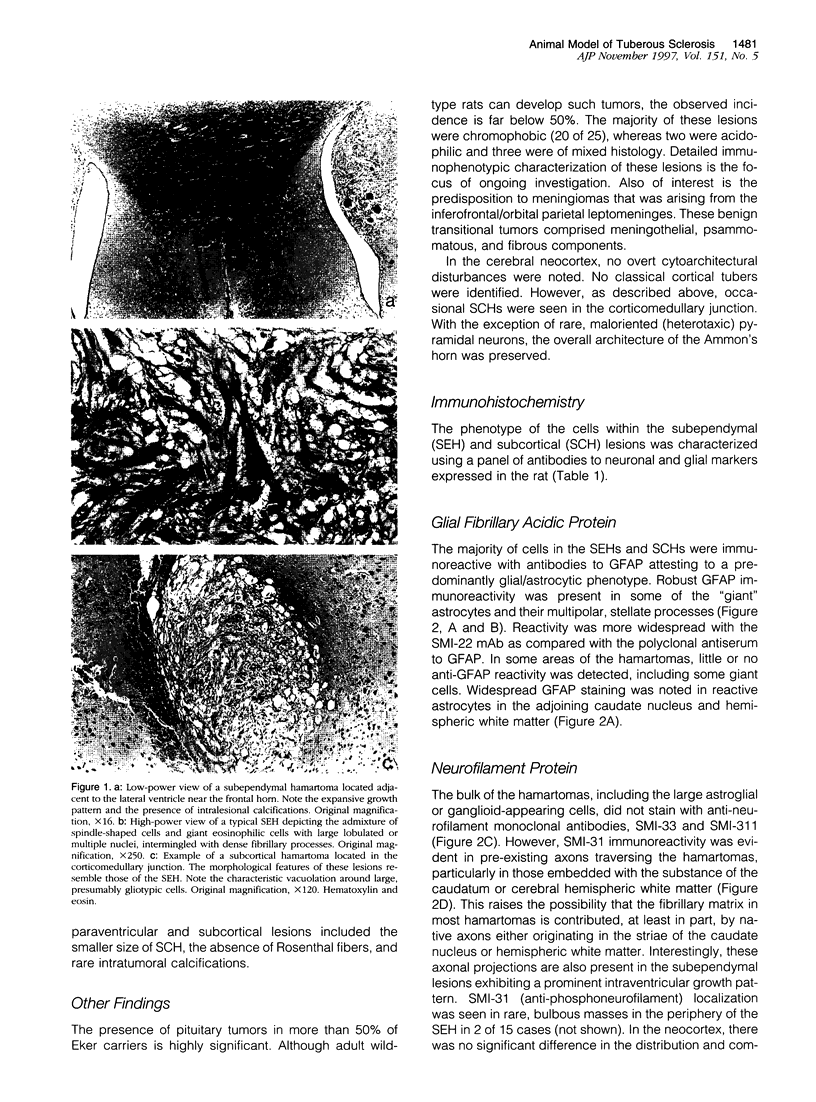
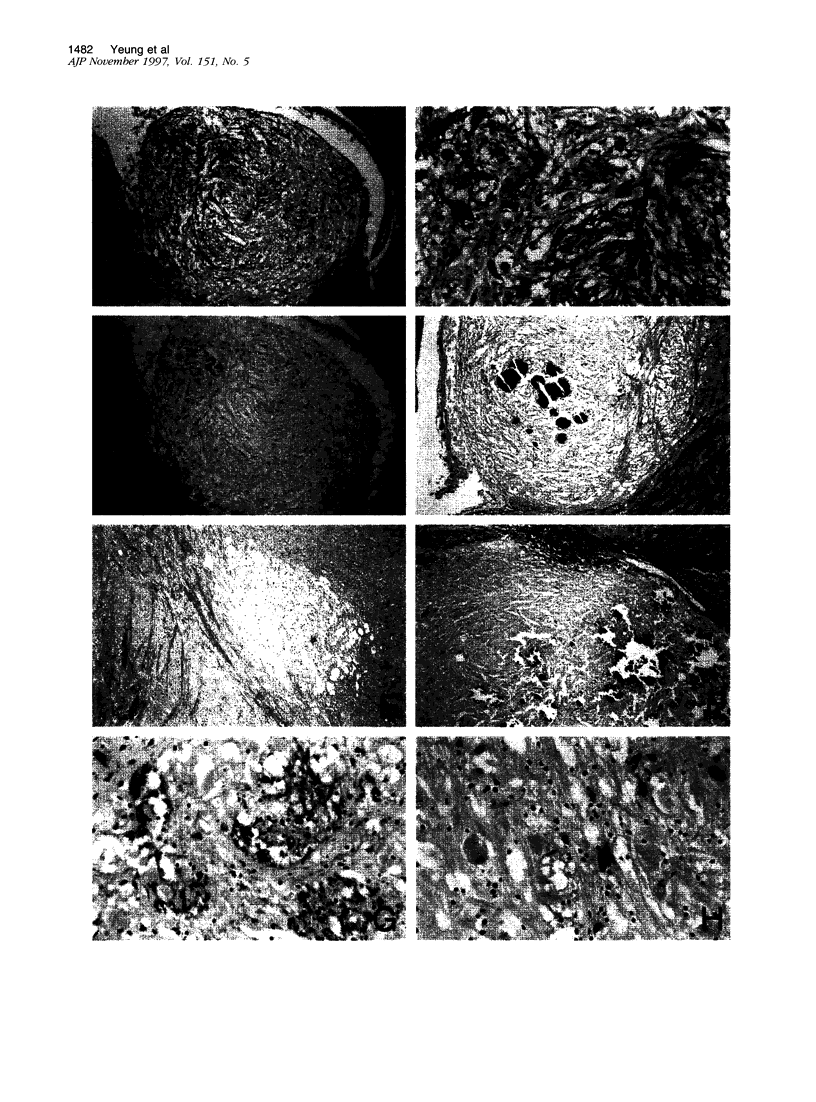
Abstract
Tuberous sclerosis (TSC) is an autosomal dominant syndrome that is linked to two genetic loci: TSC1 (9q34) and TSC2 (16p13). Brain manifestations such as cortical tubers and subependymal hamartoma/giant cell astrocytomas are major causes of TSC-related morbidity. In this study, we describe the central nervous system involvement in a unique rodent model of tuberous sclerosis. The Eker rat carries a spontaneous germline mutation of the TSC2 gene and is predisposed to multiple neoplasia. In a series of 45 adult Eker carriers (TSC2 +/-), three types of focal intracranial lesions were found, of which the subependymal and subcortical hamartomas were most prevalent (65%). There exist remarkable phenotypic similarities between the Eker rat and human subependymal lesions. Our study indicates that the predominant cellular phenotype of the subependymal hamartomas is astroglial and suggests that the neuronal contribution within these lesions is, in part, the result of pre-existing myelinated axons. The hamartomas did not show evidence of loss of the wild-type TSC2 allele; it remains to be determined whether TSC2 inactivation is necessary for their pathogenesis. This genetically-defined rodent model may be useful in elucidating the molecular and developmental basis of the subependymal giant cell astrocytoma in humans.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender B. L., Yunis E. J. Central nervous system pathology of tuberous sclerosis in children. Ultrastruct Pathol. 1980 Jul-Sep;1(3):287–299. doi: 10.3109/01913128009141432. [DOI] [PubMed] [Google Scholar]
- Bonnin J. M., Rubinstein L. J., Papasozomenos S. C., Marangos P. J. Subependymal giant cell astrocytoma. Significance and possible cytogenetic implications of an immunohistochemical study. Acta Neuropathol. 1984;62(3):185–193. doi: 10.1007/BF00691851. [DOI] [PubMed] [Google Scholar]
- Carbonara C., Longa L., Grosso E., Mazzucco G., Borrone C., Garrè M. L., Brisigotti M., Filippi G., Scabar A., Giannotti A. Apparent preferential loss of heterozygosity at TSC2 over TSC1 chromosomal region in tuberous sclerosis hamartomas. Genes Chromosomes Cancer. 1996 Jan;15(1):18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Celio M. R. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35(2):375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Cheng B., Christakos S., Mattson M. P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994 Jan;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Chou T. M., Chou S. M. Tuberous sclerosis in the premature infant: a report of a case with immunohistochemistry on the CNS. Clin Neuropathol. 1989 Jan-Feb;8(1):45–52. [PubMed] [Google Scholar]
- Crino P. B., Trojanowski J. Q., Dichter M. A., Eberwine J. Embryonic neuronal markers in tuberous sclerosis: single-cell molecular pathology. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14152–14157. doi: 10.1073/pnas.93.24.14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo P., Cusmai R., Cortesi F., Chiron C., Jambaque I., Dulac O. Neuropsychiatric aspects of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:8–16. doi: 10.1111/j.1749-6632.1991.tb37743.x. [DOI] [PubMed] [Google Scholar]
- DeClue J. E., Papageorge A. G., Fletcher J. A., Diehl S. R., Ratner N., Vass W. C., Lowy D. R. Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell. 1992 Apr 17;69(2):265–273. doi: 10.1016/0092-8674(92)90407-4. [DOI] [PubMed] [Google Scholar]
- Franke F. E., Schachenmayr W., Osborn M., Altmannsberger M. Unexpected immunoreactivities of intermediate filament antibodies in human brain and brain tumors. Am J Pathol. 1991 Jul;139(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- Green A. J., Smith M., Yates J. R. Loss of heterozygosity on chromosome 16p13.3 in hamartomas from tuberous sclerosis patients. Nat Genet. 1994 Feb;6(2):193–196. doi: 10.1038/ng0294-193. [DOI] [PubMed] [Google Scholar]
- Henske E. P., Scheithauer B. W., Short M. P., Wollmann R., Nahmias J., Hornigold N., van Slegtenhorst M., Welsh C. T., Kwiatkowski D. J. Allelic loss is frequent in tuberous sclerosis kidney lesions but rare in brain lesions. Am J Hum Genet. 1996 Aug;59(2):400–406. [PMC free article] [PubMed] [Google Scholar]
- Hirose T., Scheithauer B. W., Lopes M. B., Gerber H. A., Altermatt H. J., Hukee M. J., VandenBerg S. R., Charlesworth J. C. Tuber and subependymal giant cell astrocytoma associated with tuberous sclerosis: an immunohistochemical, ultrastructural, and immunoelectron and microscopic study. Acta Neuropathol. 1995;90(4):387–399. doi: 10.1007/BF00315012. [DOI] [PubMed] [Google Scholar]
- Jin F., Wienecke R., Xiao G. H., Maize J. C., Jr, DeClue J. E., Yeung R. S. Suppression of tumorigenicity by the wild-type tuberous sclerosis 2 (Tsc2) gene and its C-terminal region. Proc Natl Acad Sci U S A. 1996 Aug 20;93(17):9154–9159. doi: 10.1073/pnas.93.17.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsetos C. D., Burger P. C. Medulloblastoma. Semin Diagn Pathol. 1994 May;11(2):85–97. [PubMed] [Google Scholar]
- Katsetos C. D., Frankfurter A., Christakos S., Mancall E. L., Vlachos I. N., Urich H. Differential localization of class III, beta-tubulin isotype and calbindin-D28k defines distinct neuronal types in the developing human cerebellar cortex. J Neuropathol Exp Neurol. 1993 Nov;52(6):655–666. doi: 10.1097/00005072-199311000-00013. [DOI] [PubMed] [Google Scholar]
- Katsetos C. D., Herman M. M., Frankfurter A., Gass P., Collins V. P., Walker C. C., Rosemberg S., Barnard R. O., Rubinstein L. J. Cerebellar desmoplastic medulloblastomas. A further immunohistochemical characterization of the reticulin-free pale islands. Arch Pathol Lab Med. 1989 Sep;113(9):1019–1029. [PubMed] [Google Scholar]
- Katsetos C. D., Herman M. M., Krishna L., Vender J. R., Vinores S. A., Agamanolis D. P., Schiffer D., Burger P. C., Urich H. Calbindin-D28k in subsets of medulloblastomas and in the human medulloblastoma cell line D283 Med. Arch Pathol Lab Med. 1995 Aug;119(8):734–743. [PubMed] [Google Scholar]
- Kerfoot C., Wienecke R., Menchine M., Emelin J., Maize J. C., Jr, Welsh C. T., Norman M. G., DeClue J. E., Vinters H. V. Localization of tuberous sclerosis 2 mRNA and its protein product tuberin in normal human brain and in cerebral lesions of patients with tuberous sclerosis. Brain Pathol. 1996 Oct;6(4):367–375. doi: 10.1111/j.1750-3639.1996.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Hirayama Y., Kobayashi E., Kubo Y., Hino O. A germline insertion in the tuberous sclerosis (Tsc2) gene gives rise to the Eker rat model of dominantly inherited cancer. Nat Genet. 1995 Jan;9(1):70–74. doi: 10.1038/ng0195-70. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Matesic D. F. Immunohistochemical demonstration of neuron-specific enolase and microtubule-associated protein 2 in reactive astrocytes after injury in the adult forebrain. Neuroscience. 1994 May;60(1):11–16. doi: 10.1016/0306-4522(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Lopes M. B., Frankfurter A., Zientek G. M., Herman M. M. The presence of neuron-associated microtubule proteins in the human U-251 MG cell line. A comparative immunoblot and immunohistochemical study. Mol Chem Neuropathol. 1992 Dec;17(3):273–287. doi: 10.1007/BF03160016. [DOI] [PubMed] [Google Scholar]
- Povey S., Burley M. W., Attwood J., Benham F., Hunt D., Jeremiah S. J., Franklin D., Gillett G., Malas S., Robson E. B. Two loci for tuberous sclerosis: one on 9q34 and one on 16p13. Ann Hum Genet. 1994 May;58(Pt 2):107–127. doi: 10.1111/j.1469-1809.1994.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Shepherd C. W., Gomez M. R. Mortality in the Mayo Clinic Tuberous Sclerosis Complex Study. Ann N Y Acad Sci. 1991;615:375–377. doi: 10.1111/j.1749-6632.1991.tb37786.x. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Robertson D. M. Subependymal giant-cell astrocytoma. Case report with ultrastructural study. J Neurosurg. 1979 Feb;50(2):240–245. doi: 10.3171/jns.1979.50.2.0240. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. S., Metzger D. A., Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991 Apr;10(4):506–513. [PubMed] [Google Scholar]
- Wienecke R., König A., DeClue J. E. Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J Biol Chem. 1995 Jul 7;270(27):16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- Wienecke R., Maize J. C., Jr, Shoarinejad F., Vass W. C., Reed J., Bonifacino J. S., Resau J. H., de Gunzburg J., Yeung R. S., DeClue J. E. Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene. 1996 Sep 5;13(5):913–923. [PubMed] [Google Scholar]
- Xiao G. H., Jin F., Yeung R. S. Germ-line Tsc2 mutation in a dominantly inherited cancer model defines a novel family of rat intracisternal-A particle elements. Oncogene. 1995 Jul 6;11(1):81–87. [PubMed] [Google Scholar]
- Xiao G. H., Shoarinejad F., Jin F., Golemis E. A., Yeung R. S. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem. 1997 Mar 7;272(10):6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Everitt J. I., Jin F., Walker C. L. Allelic loss at the tuberous sclerosis 2 locus in spontaneous tumors in the Eker rat. Mol Carcinog. 1995 Sep;14(1):28–36. doi: 10.1002/mc.2940140107. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Jin F., Lee W. C., Testa J. R., Knudson A. G. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Kawata M., Miura Y., Musha T., Sasaki T., Kikuchi A., Takai Y. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol Cell Biol. 1992 Aug;12(8):3407–3414. doi: 10.1128/mcb.12.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zientek G. M., Herman M. M., Katsetos C. D., Frankfurter A. Absence of neuron-associated microtubule proteins in the rat C-6 glioma cell line. A comparative immunoblot and immunohistochemical study. Neuropathol Appl Neurobiol. 1993 Aug;19(4):346–349. doi: 10.1111/j.1365-2990.1993.tb00450.x. [DOI] [PubMed] [Google Scholar]





