Abstract
Microglial activation is central to the inflammatory response in Alzheimer's Disease (AD). A recently described mouse line, Tg(HuAPP695.K670N/M671L)2576, expressing human amyloid precursor protein with a familial AD gene mutation, age-related amyloid deposits, and memory deficits, was found to develop a significant microglial response using Griffonia simplicifolia lectin or phosphotyrosine probe to identify microglia Both Griffonia simplicifolia lectin and phosphotyrosine staining showed increased numbers of intensely labeled, often enlarged microglia clustered in and around plaques, consistent with microglial activation related to beta-amyloid formation. Using quantitative image analysis of coronal phosphotyrosine-immunostained sections, transgene-positive 10- to 16-month-old, hemizygous, hybrid Tg2576 (APPsw) animals showed significantly increased microglial density and size in plaque-forming areas of hippocampus and frontal, entorhinal, and occipital cortex. Quantitative analysis of microglia as a function of distance from the center of plaques (double labeled for A beta peptide and microglia) revealed highly significant, two- to fivefold elevations in microglial number and area within plaques compared with neighboring regions. Tg2576 beta-amyloid-plaque-forming mice should be a useful system for assessing the consequences of the microglial-mediated inflammatory response to beta-amyloid and developing anti-inflammatory therapeutic strategies for Alzheimer's disease. These results provide the first quantitative link between beta-amyloid plaque formation and microglial activation in an animal model with neuritic plaques and memory deficits.
Full text
PDF


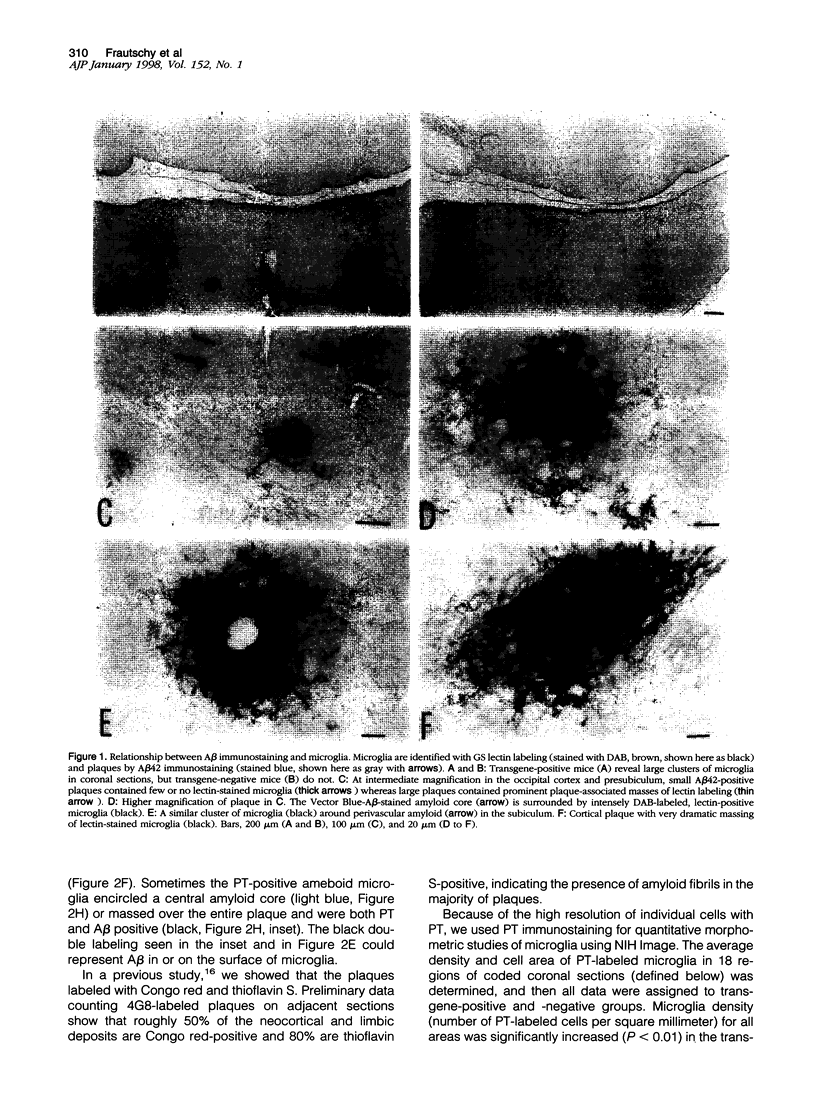
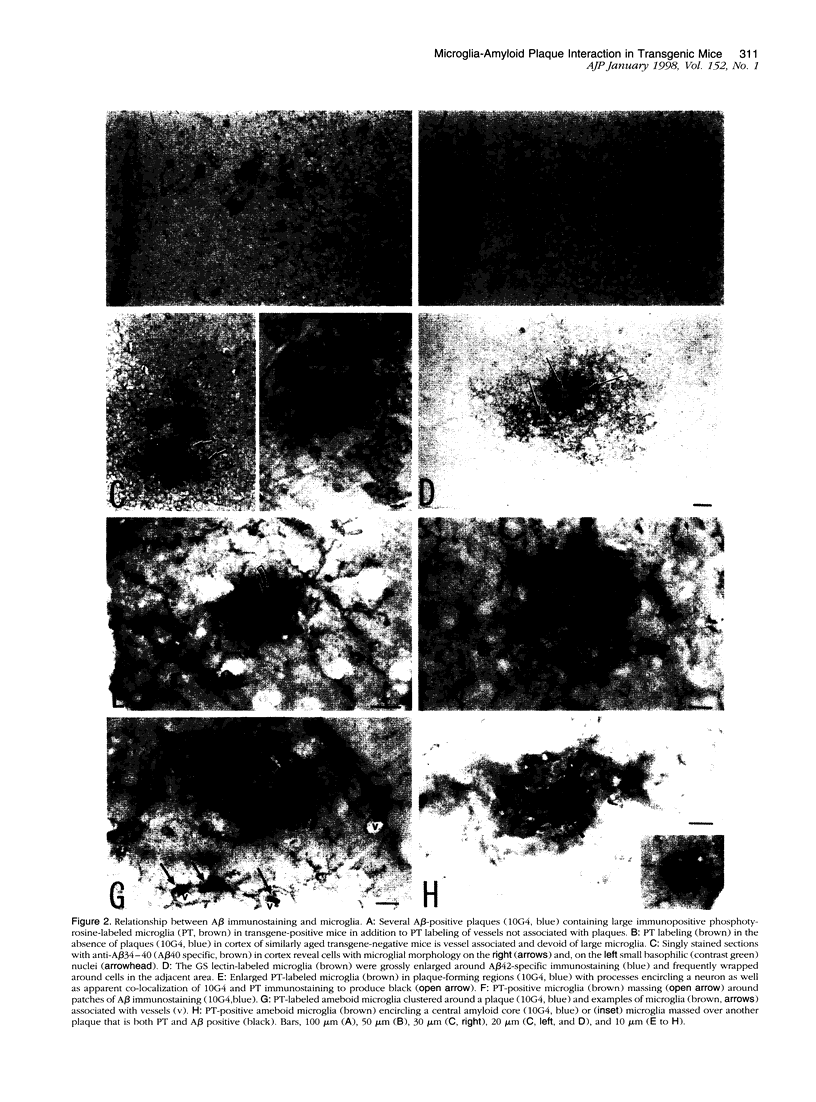

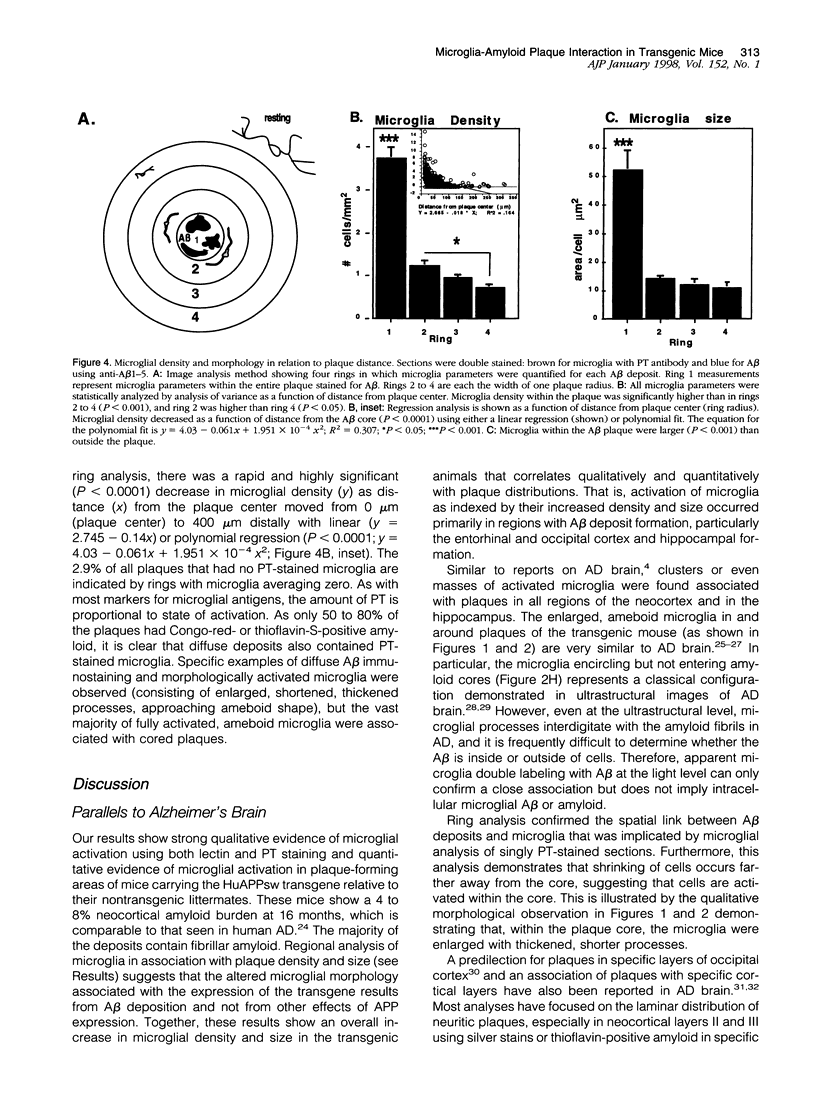




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ard M. D., Cole G. M., Wei J., Mehrle A. P., Fratkin J. D. Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res. 1996 Jan 15;43(2):190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Kalus P. Alzheimer's disease: areal and laminar pathology in the occipital isocortex. Acta Neuropathol. 1989;77(5):494–506. doi: 10.1007/BF00687251. [DOI] [PubMed] [Google Scholar]
- Cole G. M., Masliah E., Shelton E. R., Chan H. W., Terry R. D., Saitoh T. Accumulation of amyloid precursor fragment in Alzheimer plaques. Neurobiol Aging. 1991 Mar-Apr;12(2):85–91. doi: 10.1016/0197-4580(91)90046-m. [DOI] [PubMed] [Google Scholar]
- Delaère P., Duyckaerts C., He Y., Piette F., Hauw J. J. Subtypes and differential laminar distributions of beta A4 deposits in Alzheimer's disease: relationship with the intellectual status of 26 cases. Acta Neuropathol. 1991;81(3):328–335. doi: 10.1007/BF00305876. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P., Zhan S. S., van Gool W. A., Allsop D. Inflammatory mechanisms in Alzheimer's disease. Trends Pharmacol Sci. 1994 Dec;15(12):447–450. doi: 10.1016/0165-6147(94)90057-4. [DOI] [PubMed] [Google Scholar]
- El Khoury J., Hickman S. E., Thomas C. A., Cao L., Silverstein S. C., Loike J. D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996 Aug 22;382(6593):716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- Frautschy S. A., Yang F., Calderón L., Cole G. M. Rodent models of Alzheimer's disease: rat A beta infusion approaches to amyloid deposits. Neurobiol Aging. 1996 Mar-Apr;17(2):311–321. doi: 10.1016/0197-4580(95)02073-x. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Asami-Odaka A., Suzuki N., Iwatsubo T. Association of A beta 40-positive senile plaques with microglial cells in the brains of patients with Alzheimer's disease and in non-demented aged individuals. Neurodegeneration. 1996 Mar;5(1):13–17. doi: 10.1006/neur.1996.0002. [DOI] [PubMed] [Google Scholar]
- Games D., Adams D., Alessandrini R., Barbour R., Berthelette P., Blackwell C., Carr T., Clemens J., Donaldson T., Gillespie F. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995 Feb 9;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gearing M., Wilson R. W., Unger E. R., Shelton E. R., Chan H. W., Masters C. L., Beyreuther K., Mirra S. S. Amyloid precursor protein (APP) in the striatum in Alzheimer's disease: an immunohistochemical study. J Neuropathol Exp Neurol. 1993 Jan;52(1):22–30. doi: 10.1097/00005072-199301000-00004. [DOI] [PubMed] [Google Scholar]
- Giulian D., Haverkamp L. J., Yu J. H., Karshin W., Tom D., Li J., Kirkpatrick J., Kuo L. M., Roher A. E. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci. 1996 Oct 1;16(19):6021–6037. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin W. S., Sheng J. G., Roberts G. W., Mrak R. E. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J Neuropathol Exp Neurol. 1995 Mar;54(2):276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996 Oct 4;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Irizarry M. C., McNamara M., Fedorchak K., Hsiao K., Hyman B. T. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997 Sep;56(9):965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Ishii K., Tamaoka A., Mizusawa H., Shoji S., Ohtake T., Fraser P. E., Takahashi H., Tsuji S., Gearing M., Mizutani T. Abeta1-40 but not Abeta1-42 levels in cortex correlate with apolipoprotein E epsilon4 allele dosage in sporadic Alzheimer's disease. Brain Res. 1997 Feb 14;748(1-2):250–252. doi: 10.1016/s0006-8993(96)01363-7. [DOI] [PubMed] [Google Scholar]
- Johnson-Wood K., Lee M., Motter R., Hu K., Gordon G., Barbour R., Khan K., Gordon M., Tan H., Games D. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp H. L., Tillotson M. L., Soria J., Reich C., Wood J. G. Microglial tyrosine phosphorylation systems in normal and degenerating brain. Glia. 1994 Jul;11(3):284–290. doi: 10.1002/glia.440110310. [DOI] [PubMed] [Google Scholar]
- Kato H., Kogure K., Araki T., Itoyama Y. Graded expression of immunomolecules on activated microglia in the hippocampus following ischemia in a rat model of ischemic tolerance. Brain Res. 1995 Oct 2;694(1-2):85–93. doi: 10.1016/0006-8993(95)00769-m. [DOI] [PubMed] [Google Scholar]
- Kida E., Golabek A. A., Wisniewski T., Wisniewski K. E. Regional differences in apolipoprotein E immunoreactivity in diffuse plaques in Alzheimer's disease brain. Neurosci Lett. 1994 Feb 14;167(1-2):73–76. doi: 10.1016/0304-3940(94)91030-8. [DOI] [PubMed] [Google Scholar]
- Kiefer R., Streit W. J., Toyka K. V., Kreutzberg G. W., Hartung H. P. Transforming growth factor-beta 1: a lesion-associated cytokine of the nervous system. Int J Dev Neurosci. 1995 Jun-Jul;13(3-4):331–339. doi: 10.1016/0736-5748(94)00074-d. [DOI] [PubMed] [Google Scholar]
- Kimura T., Hisano T., Yoshida H., Ueda K., Miyakawa T. Re-evaluation of classic senile plaques by three-dimensional analysis. J Neurol. 1994 Oct;241(10):624–627. doi: 10.1007/BF00920628. [DOI] [PubMed] [Google Scholar]
- Klegeris A., Walker D. G., McGeer P. L. Activation of macrophages by Alzheimer beta amyloid peptide. Biochem Biophys Res Commun. 1994 Mar 15;199(2):984–991. doi: 10.1006/bbrc.1994.1326. [DOI] [PubMed] [Google Scholar]
- Korematsu K., Goto S., Nagahiro S., Ushio Y. Microglial response to transient focal cerebral ischemia: an immunocytochemical study on the rat cerebral cortex using anti-phosphotyrosine antibody. J Cereb Blood Flow Metab. 1994 Sep;14(5):825–830. doi: 10.1038/jcbfm.1994.103. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Rogers J., Kowall N. W. Senile plaques are located between apical dendritic clusters. J Neuropathol Exp Neurol. 1987 Jan;46(1):1–11. doi: 10.1097/00005072-198701000-00001. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G. W. Microglia, the first line of defence in brain pathologies. Arzneimittelforschung. 1995 Mar;45(3A):357–360. [PubMed] [Google Scholar]
- Lue L. F., Brachova L., Civin W. H., Rogers J. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J Neuropathol Exp Neurol. 1996 Oct;55(10):1083–1088. [PubMed] [Google Scholar]
- Mackenzie I. R., Hao C., Munoz D. G. Role of microglia in senile plaque formation. Neurobiol Aging. 1995 Sep-Oct;16(5):797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- Majocha R. E., Benes F. M., Reifel J. L., Rodenrys A. M., Marotta C. A. Laminar-specific distribution and infrastructural detail of amyloid in the Alzheimer disease cortex visualized by computer-enhanced imaging of epitopes recognized by monoclonal antibodies. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6182–6186. doi: 10.1073/pnas.85.16.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak K., Yang F., Vinters H. V., Frautschy S. A., Cole G. M. Polyclonals to beta-amyloid(1-42) identify most plaque and vascular deposits in Alzheimer cortex, but not striatum. Brain Res. 1994 Dec 19;667(1):138–142. doi: 10.1016/0006-8993(94)91725-6. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Iwatsubo T., Fukumoto H., Ihara Y., Odaka A., Suzuki N. Microglial cells and amyloid beta protein (A beta) deposition; association with A beta 40-containing plaques. Acta Neuropathol. 1995;90(5):472–477. doi: 10.1007/BF00294808. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Iwatsubo T., Ihara Y., Cairns N. J., Lantos P. L., Bogdanovic N., Lannfelt L., Winblad B., Maat-Schieman M. L., Rossor M. N. Predominant deposition of amyloid-beta 42(43) in plaques in cases of Alzheimer's disease and hereditary cerebral hemorrhage associated with mutations in the amyloid precursor protein gene. Am J Pathol. 1996 Apr;148(4):1257–1266. [PMC free article] [PubMed] [Google Scholar]
- Mann D. M., Iwatsubo T., Pickering-Brown S. M., Owen F., Saido T. C., Perry R. H. Preferential deposition of amyloid beta protein (Abeta) in the form Abeta40 in Alzheimer's disease is associated with a gene dosage effect of the apolipoprotein E E4 allele. Neurosci Lett. 1997 Jan 17;221(2-3):81–84. doi: 10.1016/s0304-3940(96)13294-8. [DOI] [PubMed] [Google Scholar]
- Martin L. J., Pardo C. A., Cork L. C., Price D. L. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am J Pathol. 1994 Dec;145(6):1358–1381. [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Sisk A., Mallory M., Mucke L., Schenk D., Games D. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F beta-amyloid precursor protein and Alzheimer's disease. J Neurosci. 1996 Sep 15;16(18):5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P. L., Schulzer M., McGeer E. G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology. 1996 Aug;47(2):425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Meda L., Cassatella M. A., Szendrei G. I., Otvos L., Jr, Baron P., Villalba M., Ferrari D., Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995 Apr 13;374(6523):647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki A., Peterson J. W., Mufson E. J., Trapp B. D. Amyloid load and neural elements in Alzheimer's disease and nondemented individuals with high amyloid plaque density. Exp Neurol. 1996 Nov;142(1):89–102. doi: 10.1006/exnr.1996.0181. [DOI] [PubMed] [Google Scholar]
- Paresce D. M., Ghosh R. N., Maxfield F. R. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron. 1996 Sep;17(3):553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Burdick D., Walencewicz A. J., Glabe C. G., Cotman C. W. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993 Apr;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst A., Langui D., Ipsen S., Robakis N., Ulrich J. Deposition of beta/A4 protein along neuronal plasma membranes in diffuse senile plaques. Acta Neuropathol. 1991;83(1):21–29. doi: 10.1007/BF00294426. [DOI] [PubMed] [Google Scholar]
- Rafalowska J., Barcikowska M., Wen G. Y., Wisniewski H. M. Laminar distribution of neuritic plaques in normal aging, Alzheimer's disease and Down's syndrome. Acta Neuropathol. 1988;77(1):21–25. doi: 10.1007/BF00688238. [DOI] [PubMed] [Google Scholar]
- Roe M. T., Dawson D. V., Hulette C. M., Einstein G., Crain B. J. Microglia are not exclusively associated with plaque-rich regions of the dentate gyrus in Alzheimer's disease. J Neuropathol Exp Neurol. 1996 Mar;55(3):366–371. doi: 10.1097/00005072-199603000-00012. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Bots G. T., Roos R. A., Eikelenboom P. Acute phase proteins but not activated microglial cells are present in parenchymal beta/A4 deposits in the brains of patients with hereditary cerebral hemorrhage with amyloidosis-Dutch type. Neurosci Lett. 1992 Jun 22;140(2):137–140. doi: 10.1016/0304-3940(92)90087-n. [DOI] [PubMed] [Google Scholar]
- Saido T. C., Iwatsubo T., Mann D. M., Shimada H., Ihara Y., Kawashima S. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995 Feb;14(2):457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994 Sep;53(5):438–447. doi: 10.1097/00005072-199409000-00003. [DOI] [PubMed] [Google Scholar]
- Shaffer L. M., Dority M. D., Gupta-Bansal R., Frederickson R. C., Younkin S. G., Brunden K. R. Amyloid beta protein (A beta) removal by neuroglial cells in culture. Neurobiol Aging. 1995 Sep-Oct;16(5):737–745. doi: 10.1016/0197-4580(95)00055-j. [DOI] [PubMed] [Google Scholar]
- Sheng J. G., Mrak R. E., Griffin W. S. Apolipoprotein E distribution among different plaque types in Alzheimer's disease: implications for its role in plaque progression. Neuropathol Appl Neurobiol. 1996 Aug;22(4):334–341. doi: 10.1111/j.1365-2990.1996.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Stewart W. F., Kawas C., Corrada M., Metter E. J. Risk of Alzheimer's disease and duration of NSAID use. Neurology. 1997 Mar;48(3):626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Uchihara T., Duyckaerts C., He Y., Kobayashi K., Seilhean D., Amouyel P., Hauw J. J. ApoE immunoreactivity and microglial cells in Alzheimer's disease brain. Neurosci Lett. 1995 Jul 28;195(1):5–8. doi: 10.1016/0304-3940(95)11763-m. [DOI] [PubMed] [Google Scholar]
- Uchihara T., Kondo H., Akiyama H., Ikeda K. White matter amyloid in Alzheimer's disease brain. Acta Neuropathol. 1995;90(1):51–56. doi: 10.1007/BF00294459. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J. The role of microglia in amyloid fibril formation. Neuropathol Appl Neurobiol. 1994 Apr;20(2):192–194. [PubMed] [Google Scholar]
- Wisniewski H. M., Wegiel J., Wang K. C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can J Neurol Sci. 1989 Nov;16(4 Suppl):535–542. doi: 10.1017/s0317167100029887. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996 Aug 22;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yang F., Mak K., Vinters H. V., Frautschy S. A., Cole G. M. Monoclonal antibody to the C-terminus of beta-amyloid. Neuroreport. 1994 Oct 27;5(16):2117–2120. doi: 10.1097/00001756-199410270-00032. [DOI] [PubMed] [Google Scholar]