Abstract
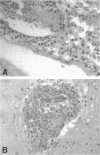
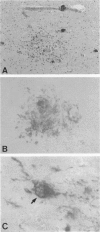
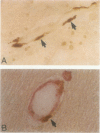
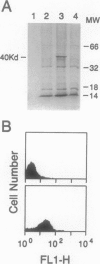
The chemokine receptors CCR5 and CXCR4 are co-receptors together with CD4 for human immunodeficiency virus (HIV)-1 entry into target cells. Macrophage-tropic HIV-1 viruses use CCR5 as a co-receptor, whereas T-cell-line tropic viruses use CXCR4. HIV-1 infects the brain and causes a progressive encephalopathy in 20 to 30% of infected children and adults. Most of the HIV-1-infected cells in the brain are macrophages and microglia. We examined expression of CCR5 and CXCR4 in brain tissue from 20 pediatric acquired immune deficiency syndrome (AIDS) patients in relation to neuropathological consequences of HIV-1 infection. The overall frequency of CCR5-positive perivascular mononuclear cells and macrophages was increased in the brains of children with severe HIV-1 encephalitis (HIVE) compared with children with mild HIVE or non-AIDS controls, whereas the frequency of CXCR4-positive perivascular cells did not correlate with disease severity. CCR5- and CXCR4-positive macrophages and microglia were detected in inflammatory lesions in the brain of children with severe HIVE. In addition, CXCR4 was detected in a subpopulation of neurons in autopsy brain tissue and primary human brain cultures. Similar findings were demonstrated in the brain of adult AIDS patients and controls. These findings suggest that CCR5-positive mononuclear cells, macrophages, and microglia contribute to disease progression in the central nervous system of children and adults with AIDS by serving as targets for virus replication.
Full text
PDF


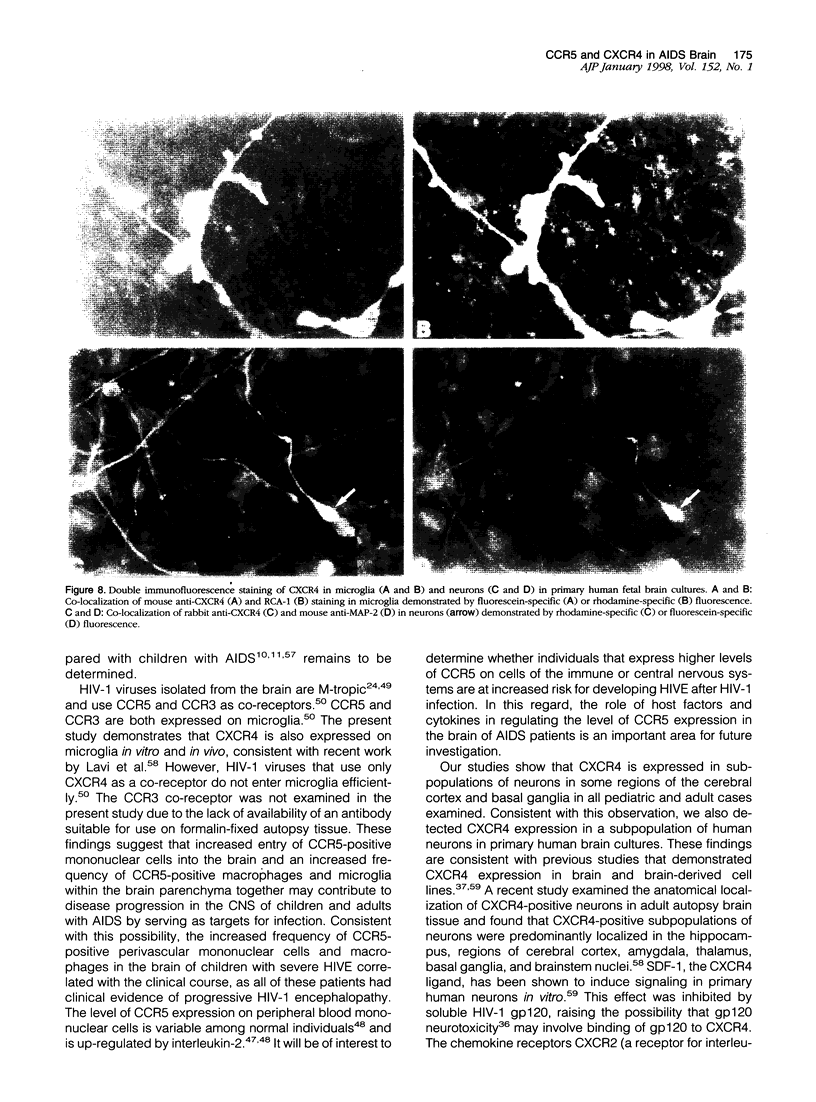



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson D. C., Dawson T. M., Zink M. C., Clements J. E., Dawson V. L. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996 Jul;2(4):417–428. [PMC free article] [PubMed] [Google Scholar]
- Adle-Biassette H., Levy Y., Colombel M., Poron F., Natchev S., Keohane C., Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995 Jun;21(3):218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Alkhatib G., Combadiere C., Broder C. C., Feng Y., Kennedy P. E., Murphy P. M., Berger E. A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996 Jun 28;272(5270):1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- An S. F., Giometto B., Scaravilli T., Tavolato B., Gray F., Scaravilli F. Programmed cell death in brains of HIV-1-positive AIDS and pre-AIDS patients. Acta Neuropathol. 1996;91(2):169–173. doi: 10.1007/s004010050409. [DOI] [PubMed] [Google Scholar]
- Bagasra O., Lavi E., Bobroski L., Khalili K., Pestaner J. P., Tawadros R., Pomerantz R. J. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996 Jun;10(6):573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Belman A. L., Ultmann M. H., Horoupian D., Novick B., Spiro A. J., Rubinstein A., Kurtzberg D., Cone-Wesson B. Neurological complications in infants and children with acquired immune deficiency syndrome. Ann Neurol. 1985 Nov;18(5):560–566. doi: 10.1002/ana.410180509. [DOI] [PubMed] [Google Scholar]
- Bleul C. C., Wu L., Hoxie J. A., Springer T. A., Mackay C. R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen D. E. Neural apoptosis. Ann Neurol. 1995 Dec;38(6):839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- Choe H., Farzan M., Sun Y., Sullivan N., Rollins B., Ponath P. D., Wu L., Mackay C. R., LaRosa G., Newman W. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996 Jun 28;85(7):1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- Connor R. I., Sheridan K. E., Ceradini D., Choe S., Landau N. R. Change in coreceptor use correlates with disease progression in HIV-1--infected individuals. J Exp Med. 1997 Feb 17;185(4):621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Liu R., Ellmeier W., Choe S., Unutmaz D., Burkhart M., Di Marzio P., Marmon S., Sutton R. E., Hill C. M. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996 Jun 20;381(6584):661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- Dickover R. E., Dillon M., Gillette S. G., Deveikis A., Keller M., Plaeger-Marshall S., Chen I., Diagne A., Stiehm E. R., Bryson Y. Rapid increases in load of human immunodeficiency virus correlate with early disease progression and loss of CD4 cells in vertically infected infants. J Infect Dis. 1994 Nov;170(5):1279–1284. doi: 10.1093/infdis/170.5.1279. [DOI] [PubMed] [Google Scholar]
- Doranz B. J., Rucker J., Yi Y., Smyth R. J., Samson M., Peiper S. C., Parmentier M., Collman R. G., Doms R. W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996 Jun 28;85(7):1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- Dragic T., Litwin V., Allaway G. P., Martin S. R., Huang Y., Nagashima K. A., Cayanan C., Maddon P. J., Koup R. A., Moore J. P. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996 Jun 20;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Endres M. J., Clapham P. R., Marsh M., Ahuja M., Turner J. D., McKnight A., Thomas J. F., Stoebenau-Haggarty B., Choe S., Vance P. J. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996 Nov 15;87(4):745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Goudsmit J. Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann Neurol. 1988;23 (Suppl):S19–S23. doi: 10.1002/ana.410230709. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Sharer L. R., Oleske J. M., Connor E. M., Goudsmit J., Bagdon L., Robert-Guroff M., Koenigsberger M. R. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986 Oct;78(4):678–687. [PubMed] [Google Scholar]
- Feng Y., Broder C. C., Kennedy P. E., Berger E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May 10;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Fuks Z., Persaud R. S., Alfieri A., McLoughlin M., Ehleiter D., Schwartz J. L., Seddon A. P., Cordon-Cardo C., Haimovitz-Friedman A. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994 May 15;54(10):2582–2590. [PubMed] [Google Scholar]
- Gabuzda D. H., Ho D. D., de la Monte S. M., Hirsch M. S., Rota T. R., Sobel R. A. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986 Sep;20(3):289–295. doi: 10.1002/ana.410200304. [DOI] [PubMed] [Google Scholar]
- Gelbard H. A., James H. J., Sharer L. R., Perry S. W., Saito Y., Kazee A. M., Blumberg B. M., Epstein L. G. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995 Jun;21(3):208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Glass J. D., Fedor H., Wesselingh S. L., McArthur J. C. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995 Nov;38(5):755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Glass J. D., Wesselingh S. L., Selnes O. A., McArthur J. C. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993 Nov;43(11):2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Gray F., Lescs M. C., Keohane C., Paraire F., Marc B., Durigon M., Gherardi R. Early brain changes in HIV infection: neuropathological study of 11 HIV seropositive, non-AIDS cases. J Neuropathol Exp Neurol. 1992 Mar;51(2):177–185. doi: 10.1097/00005072-199203000-00007. [DOI] [PubMed] [Google Scholar]
- He J., Chen Y., Farzan M., Choe H., Ohagen A., Gartner S., Busciglio J., Yang X., Hofmann W., Newman W. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997 Feb 13;385(6617):645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J., Halks-Miller M., DelVecchio V., Peiper S. C., Hoxie J., Kolson D. L., Taub D., Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997 Feb 1;7(2):112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- Horuk R., Martin A. W., Wang Z., Schweitzer L., Gerassimides A., Guo H., Lu Z., Hesselgesser J., Perez H. D., Kim J. Expression of chemokine receptors by subsets of neurons in the central nervous system. J Immunol. 1997 Mar 15;158(6):2882–2890. [PubMed] [Google Scholar]
- Huang Y., Paxton W. A., Wolinsky S. M., Neumann A. U., Zhang L., He T., Kang S., Ceradini D., Jin Z., Yazdanbakhsh K. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996 Nov;2(11):1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Ketzler S., Weis S., Haug H., Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. 1990;80(1):92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- Korber B. T., Kunstman K. J., Patterson B. K., Furtado M., McEvilly M. M., Levy R., Wolinsky S. M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994 Nov;68(11):7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure K., Llena J. F., Lyman W. D., Soeiro R., Weidenheim K. M., Hirano A., Dickson D. W. Human immunodeficiency virus-1 infection of the nervous system: an autopsy study of 268 adult, pediatric, and fetal brains. Hum Pathol. 1991 Jul;22(7):700–710. doi: 10.1016/0046-8177(91)90293-x. [DOI] [PubMed] [Google Scholar]
- Lavi E., Strizki J. M., Ulrich A. M., Zhang W., Fu L., Wang Q., O'Connor M., Hoxie J. A., González-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997 Oct;151(4):1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Gendelman H. E. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995 Apr 6;332(14):934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Liu R., Paxton W. A., Choe S., Ceradini D., Martin S. R., Horuk R., MacDonald M. E., Stuhlmann H., Koup R. A., Landau N. R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996 Aug 9;86(3):367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Moses A. V., Bloom F. E., Pauza C. D., Nelson J. A. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelcu I. AIDS in Romania. Am J Med Sci. 1992 Sep;304(3):188–191. doi: 10.1097/00000441-199209000-00008. [DOI] [PubMed] [Google Scholar]
- Nottet H. S., Persidsky Y., Sasseville V. G., Nukuna A. N., Bock P., Zhai Q. H., Sharer L. R., McComb R. D., Swindells S., Soderland C. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996 Feb 1;156(3):1284–1295. [PubMed] [Google Scholar]
- Persidsky Y., Stins M., Way D., Witte M. H., Weinand M., Kim K. S., Bock P., Gendelman H. E., Fiala M. A model for monocyte migration through the blood-brain barrier during HIV-1 encephalitis. J Immunol. 1997 Apr 1;158(7):3499–3510. [PubMed] [Google Scholar]
- Petito C. K., Cash K. S. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992 Nov;32(5):658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- Petito C. K., Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995 May;146(5):1121–1130. [PMC free article] [PubMed] [Google Scholar]
- Pizzo P. A., Wilfert C. M. Markers and determinants of disease progression in children with HIV infection. The Pediatric AIDS Siena Workshop II. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Jan 1;8(1):30–44. [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C., Kong P. A., Crawford T. O., Wesselingh S., Glass J. D., McArthur J. C., Trapp B. D. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993 Sep;34(3):339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Ranki A., Nyberg M., Ovod V., Haltia M., Elovaara I., Raininko R., Haapasalo H., Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. AIDS. 1995 Sep;9(9):1001–1008. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sharer L. R., Epstein L. G., Michaels J., Mintz M., Louder M., Golding K., Cvetkovich T. A., Blumberg B. M. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994 Mar;44(3 Pt 1):474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Samson M., Libert F., Doranz B. J., Rucker J., Liesnard C., Farber C. M., Saragosti S., Lapoumeroulie C., Cognaux J., Forceille C. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996 Aug 22;382(6593):722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H., Nottet H. S., Nuovo G., Raabe T., Flanagan C. R., Dubrovsky L., Gendelman H. E., Cerami A., Bukrinsky M., Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. B., Hutto C., Makuch R. W., Mastrucci M. T., O'Connor T., Mitchell C. D., Trapido E. J., Parks W. P. Survival in children with perinatally acquired human immunodeficiency virus type 1 infection. N Engl J Med. 1989 Dec 28;321(26):1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Shearer W. T., Quinn T. C., LaRussa P., Lew J. F., Mofenson L., Almy S., Rich K., Handelsman E., Diaz C., Pagano M. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. Women and Infants Transmission Study Group. N Engl J Med. 1997 May 8;336(19):1337–1342. doi: 10.1056/NEJM199705083361901. [DOI] [PubMed] [Google Scholar]
- Shi B., De Girolami U., He J., Wang S., Lorenzo A., Busciglio J., Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996 Nov 1;98(9):1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. W., DeGirolami U., Hénin D., Bolgert F., Hauw J. J. Human immunodeficiency virus (HIV) leukoencephalopathy and the microcirculation. J Neuropathol Exp Neurol. 1990 Jul;49(4):357–370. doi: 10.1097/00005072-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Wesselingh S. L., Griffin D. E., McArthur J. C., Johnson R. T., Glass J. D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996 Jun;39(6):705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Chandra R., Berger J. R., Major E. O. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology. 1994 Mar;44(3 Pt 1):481–487. doi: 10.1212/wnl.44.3_part_1.481. [DOI] [PubMed] [Google Scholar]
- Vazeux R., Lacroix-Ciaudo C., Blanche S., Cumont M. C., Henin D., Gray F., Boccon-Gibod L., Tardieu M. Low levels of human immunodeficiency virus replication in the brain tissue of children with severe acquired immunodeficiency syndrome encephalopathy. Am J Pathol. 1992 Jan;140(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Belman A. L., Dickson D. W., Rubinstein A., Nelson J. A. Human immunodeficiency virus within the brains of children with AIDS. Clin Neuropathol. 1990 Jan-Feb;9(1):1–6. [PubMed] [Google Scholar]
- Wiley C. A., Masliah E., Morey M., Lemere C., DeTeresa R., Grafe M., Hansen L., Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991 Jun;29(6):651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Paxton W. A., Kassam N., Ruffing N., Rottman J. B., Sullivan N., Choe H., Sodroski J., Newman W., Koup R. A. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997 May 5;185(9):1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazopoulos E., Haseltine W. A. Effect of nef alleles on replication of human immunodeficiency virus type 1. Virology. 1993 May;194(1):20–27. doi: 10.1006/viro.1993.1230. [DOI] [PubMed] [Google Scholar]