Abstract
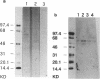
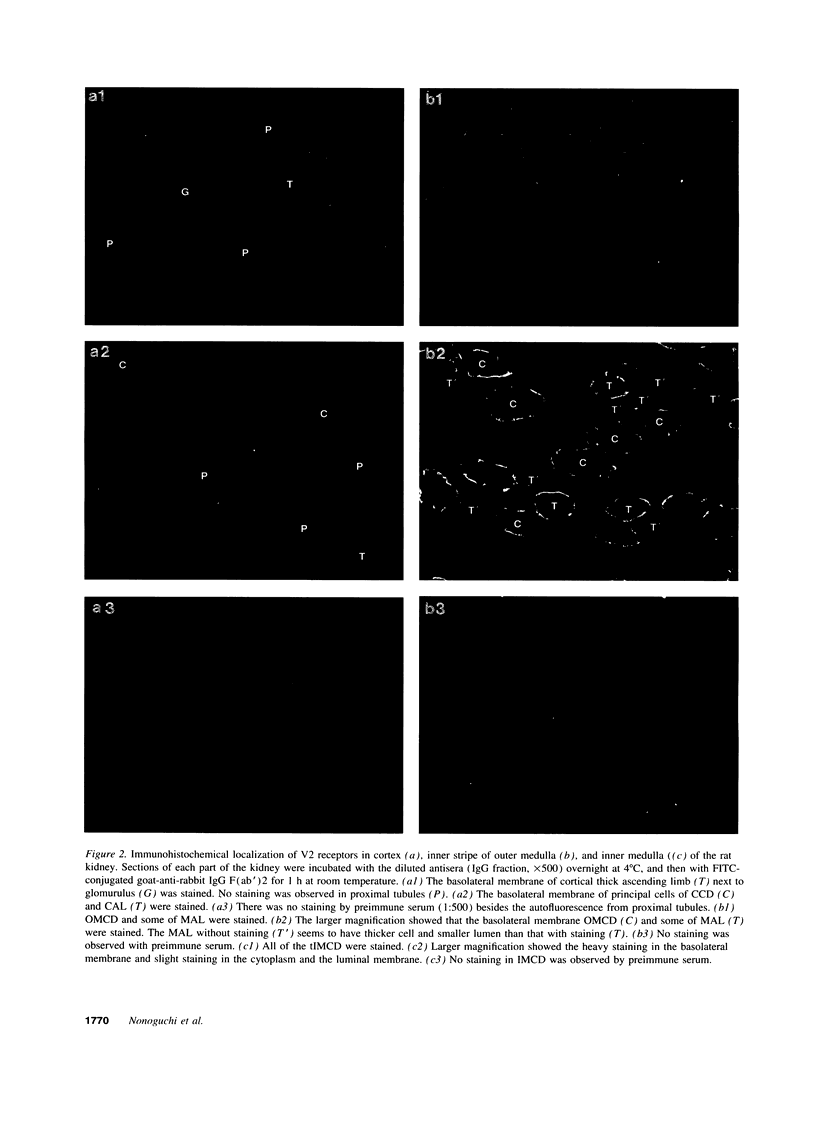
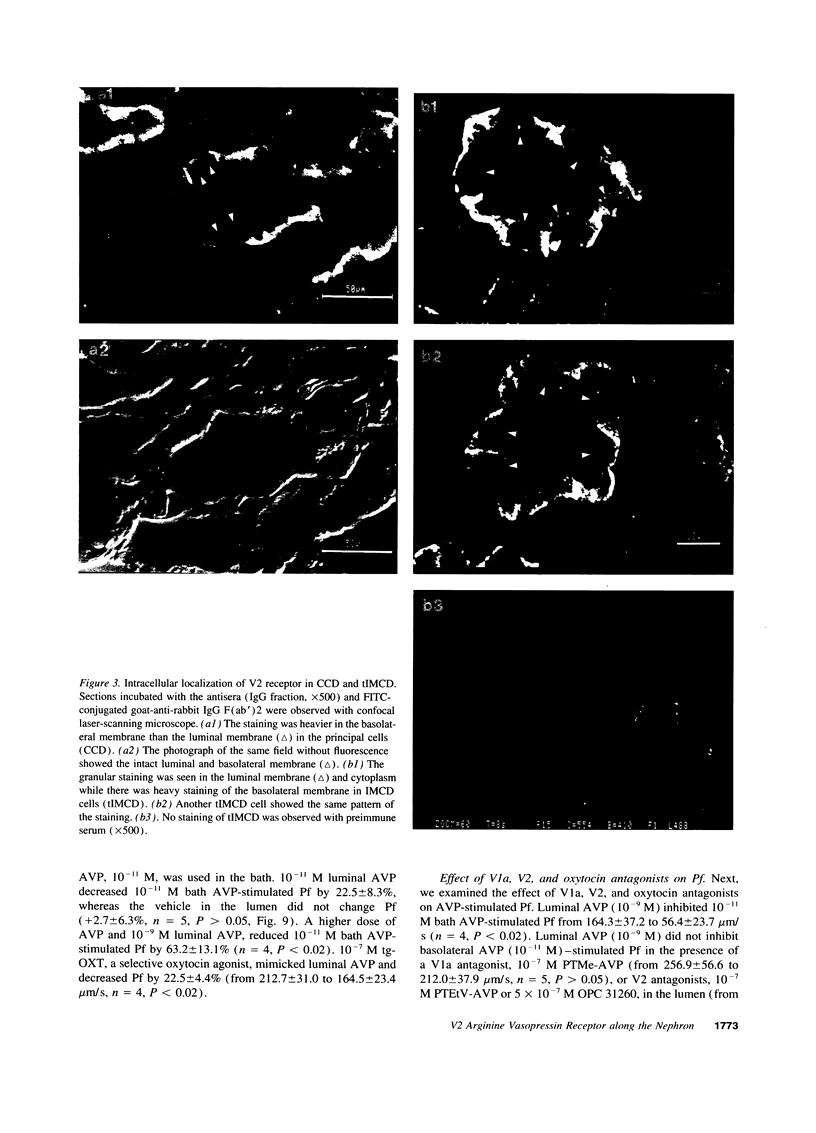
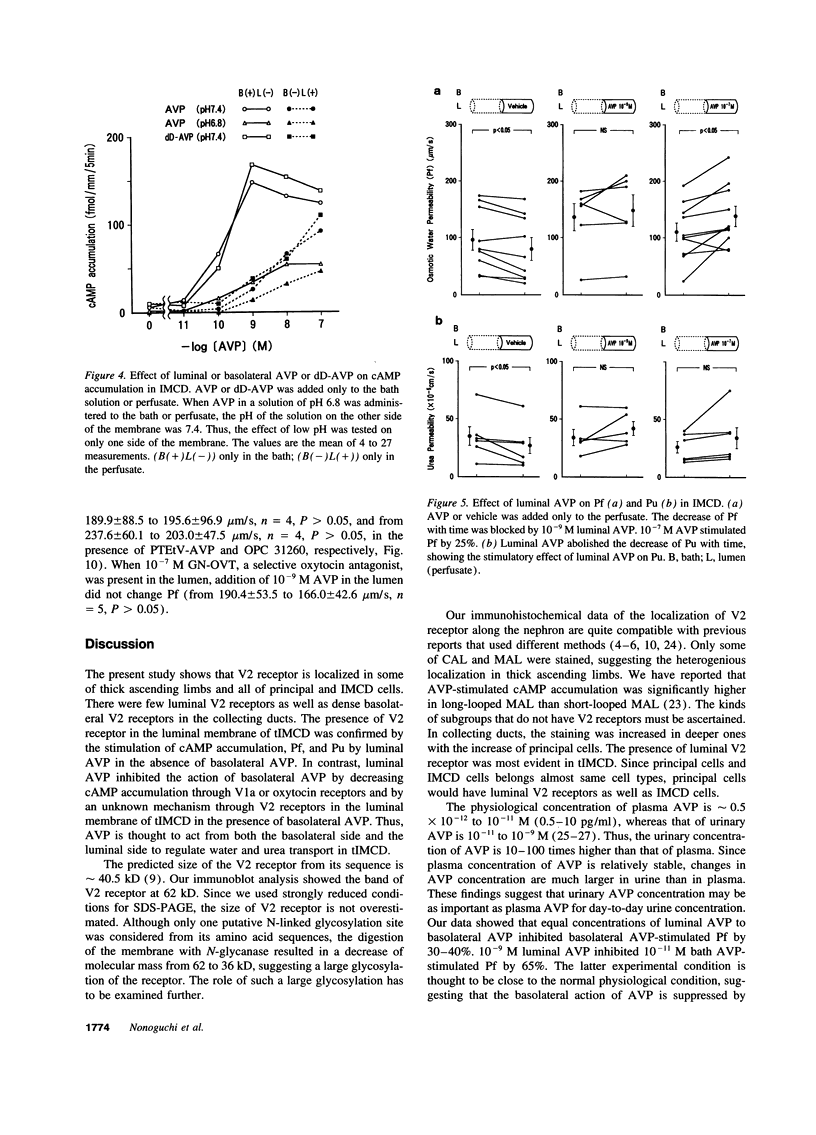
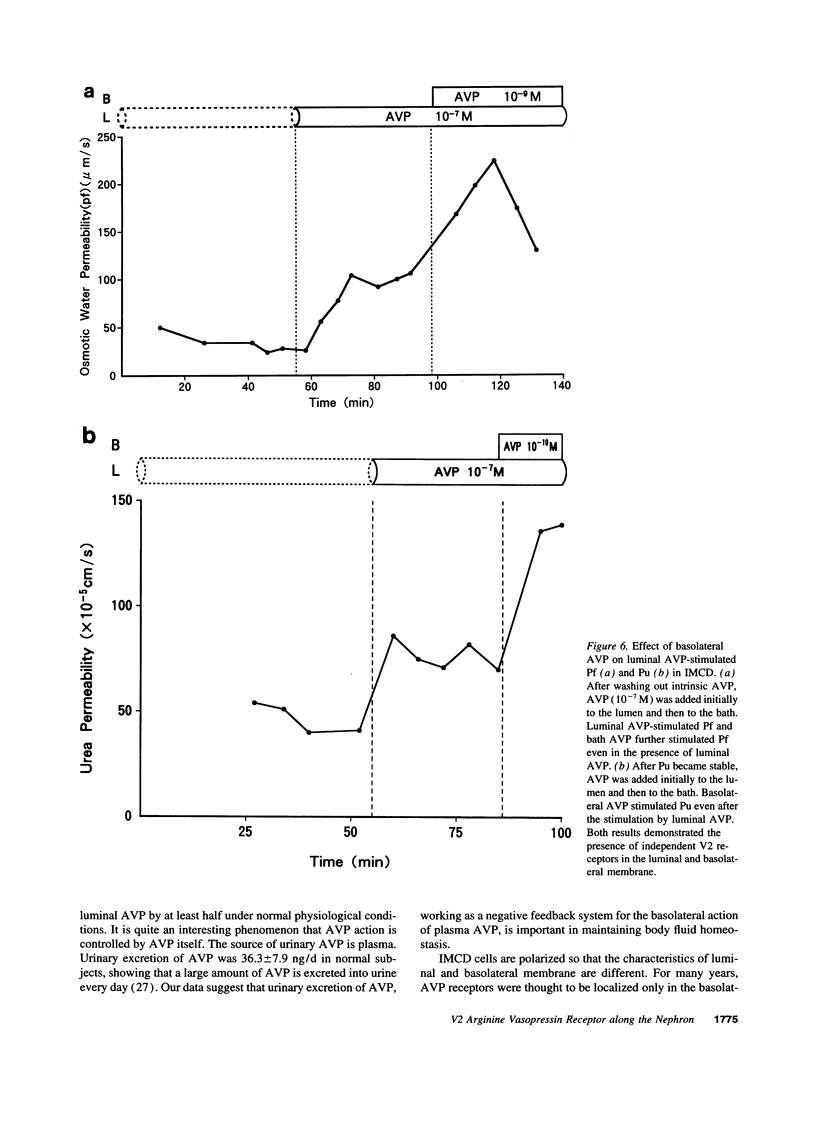
We investigated immunohistochemical localization of V2 vasopressin receptor along the nephron using a specific polyclonal antibody. Staining was observed in some of thick ascending limbs and all of principal and inner medullary collecting duct (IMCD) cells. Not only basolateral but also luminal membrane was stained in collecting ducts, especially in terminal IMCD (tIMCD). To learn the functional role of luminal V2 receptor in tIMCD, we studied the luminal effects of arginine vasopressin (AVP) on osmotic water permeability (Pf), urea permeability (Pu), and cAMP accumulation using isolated perfused rat tIMCD. In the absence of bath AVP, luminal AVP caused a small increase in cAMP accumulation, Pf and Pu, confirming the presence of V2 receptor in the lumen of tIMCD. In contrast, luminal AVP inhibited Pf and Pu by 30-65% in the presence of bath AVP by decreasing cAMP accumulation via V1a or oxytocin receptors and by an unknown mechanism via V2 receptors in the luminal membrane of tIMCD. These data show that V2 receptors are localized not only in the basolateral membrane but also in the luminal membrane of the distal nephron. Luminal AVP acts as a negative feedback system upon the basolateral action of AVP in tIMCD.
Full text
PDF
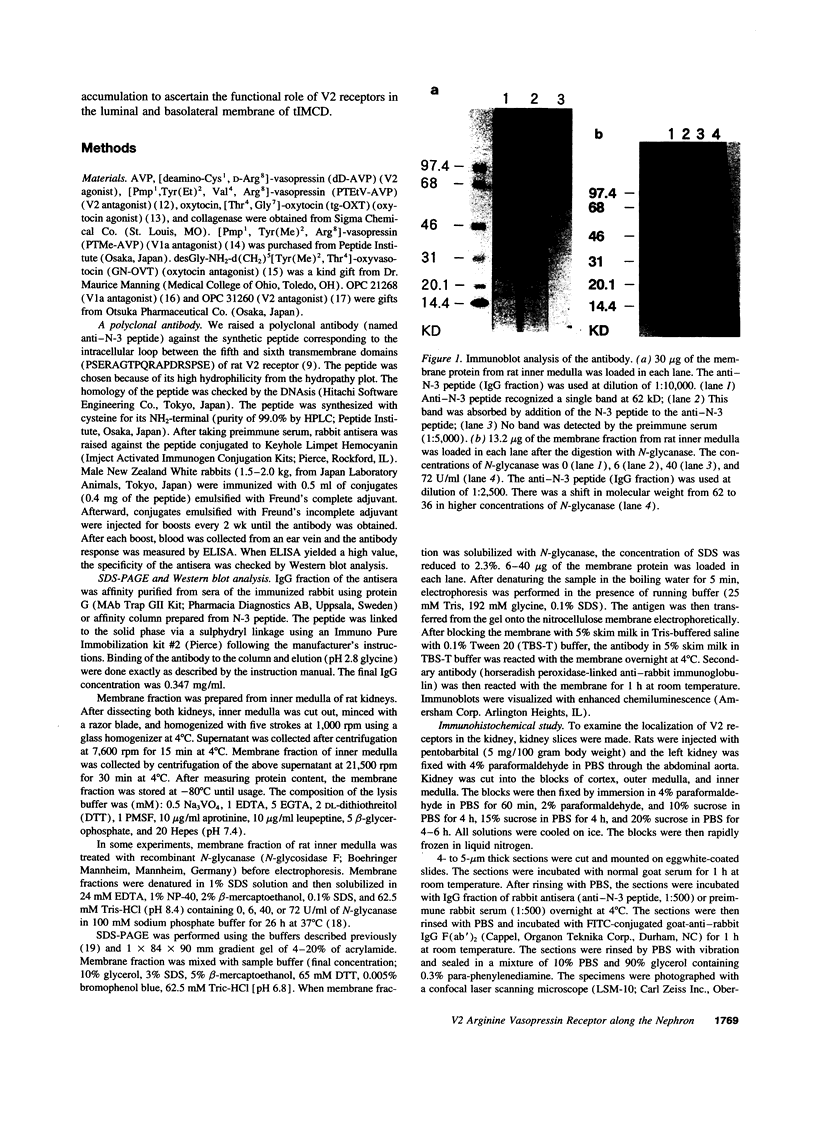




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Ando Y., Tabei K., Asano Y. Luminal vasopressin modulates transport in the rabbit cortical collecting duct. J Clin Invest. 1991 Sep;88(3):952–959. doi: 10.1172/JCI115398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner V. A., Williams B., Tsai P., Schrier R. W. Demonstration of processing and recycling of biologically active V1 vasopressin receptors in vascular smooth muscle. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2854–2858. doi: 10.1073/pnas.89.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Dickey B. F., Bucher N. L., Fine R. E. Internalization, recycling, and redistribution of vasopressin receptors in rat hepatocytes. J Biol Chem. 1985 Oct 15;260(23):12641–12646. [PubMed] [Google Scholar]
- Fressinaud P., Corvol P., Menard J. Radioimmunoassay of urinary antidiuretic hormone in man: stimulation-suppression tests. Kidney Int. 1974 Sep;6(3):184–190. doi: 10.1038/ki.1974.97. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Han J. S., Maeda Y., Knepper M. A. Dual actions of vasopressin and oxytocin in regulation of water permeability in terminal collecting duct. Am J Physiol. 1993 Jul;265(1 Pt 2):F26–F34. doi: 10.1152/ajprenal.1993.265.1.F26. [DOI] [PubMed] [Google Scholar]
- Kirk K. L. Binding and internalization of a fluorescent vasopressin analogue by collecting duct cells. Am J Physiol. 1988 Nov;255(5 Pt 1):C622–C632. doi: 10.1152/ajpcell.1988.255.5.C622. [DOI] [PubMed] [Google Scholar]
- Kruszynski M., Lammek B., Manning M., Seto J., Haldar J., Sawyer W. H. [1-beta-Mercapto-beta,beta-cyclopentamethylenepropionic acid),2-(O-methyl)tyrosine ]argine-vasopressin and [1-beta-mercapto-beta,beta-cyclopentamethylenepropionic acid)]argine-vasopressine, two highly potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1980 Apr;23(4):364–368. doi: 10.1021/jm00178a003. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Lutz W., Salisbury J. L., Kumar R. Vasopressin receptor-mediated endocytosis: current view. Am J Physiol. 1991 Jul;261(1 Pt 2):F1–13. doi: 10.1152/ajprenal.1991.261.1.F1. [DOI] [PubMed] [Google Scholar]
- Lutz W., Sanders M., Salisbury J., Kumar R. Internalization of vasopressin analogs in kidney and smooth muscle cells: evidence for receptor-mediated endocytosis in cells with V2 or V1 receptors. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6507–6511. doi: 10.1073/pnas.87.17.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M., Kruszynski M., Bankowski K., Olma A., Lammek B., Cheng L. L., Klis W. A., Seto J., Haldar J., Sawyer W. H. Solid-phase synthesis of 16 potent (selective and nonselective) in vivo antagonists of oxytocin. J Med Chem. 1989 Feb;32(2):382–391. doi: 10.1021/jm00122a016. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Morel A., O'Carroll A. M., Brownstein M. J., Lolait S. J. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992 Apr 9;356(6369):523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Morel F. Sites of hormone action in the mammalian nephron. Am J Physiol. 1981 Mar;240(3):F159–F164. doi: 10.1152/ajprenal.1981.240.3.F159. [DOI] [PubMed] [Google Scholar]
- Moses A. M., Steciak E. Urinary and metabolic clearances of arginine vasopressin in normal subjects. Am J Physiol. 1986 Aug;251(2 Pt 2):R365–R370. doi: 10.1152/ajpregu.1986.251.2.R365. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Simister N. E. Transcytosis. Cell. 1985 Dec;43(2 Pt 1):389–390. doi: 10.1016/0092-8674(85)90166-7. [DOI] [PubMed] [Google Scholar]
- Nonoguchi H., Knepper M. A., Manganiello V. C. Effects of atrial natriuretic factor on cyclic guanosine monophosphate and cyclic adenosine monophosphate accumulation in microdissected nephron segments from rats. J Clin Invest. 1987 Feb;79(2):500–507. doi: 10.1172/JCI112840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoguchi H., Sands J. M., Knepper M. A. Atrial natriuretic factor inhibits vasopressin-stimulated osmotic water permeability in rat inner medullary collecting duct. J Clin Invest. 1988 Oct;82(4):1383–1390. doi: 10.1172/JCI113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoguchi H., Tomita K., Marumo F. Effects of atrial natriuretic peptide and vasopressin on chloride transport in long- and short-looped medullary thick ascending limbs. J Clin Invest. 1992 Aug;90(2):349–357. doi: 10.1172/JCI115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi R., Nonoguchi H., Tomita K., Marumo F. Endothelin-1 inhibits AVP-stimulated osmotic water permeability in rat inner medullary collecting duct. Am J Physiol. 1991 Dec;261(6 Pt 2):F951–F956. doi: 10.1152/ajprenal.1991.261.6.F951. [DOI] [PubMed] [Google Scholar]
- Ostrowski N. L., Young W. S., 3rd, Knepper M. A., Lolait S. J. Expression of vasopressin V1a and V2 receptor messenger ribonucleic acid in the liver and kidney of embryonic, developing, and adult rats. Endocrinology. 1993 Oct;133(4):1849–1859. doi: 10.1210/endo.133.4.8404628. [DOI] [PubMed] [Google Scholar]
- Phillips P. A., Abrahams J. M., Kelly J. M., Mooser V., Trinder D., Johnston C. I. Localization of vasopressin binding sites in rat tissues using specific V1 and V2 selective ligands. Endocrinology. 1990 Mar;126(3):1478–1484. doi: 10.1210/endo-126-3-1478. [DOI] [PubMed] [Google Scholar]
- Pruszczynski W., Caillens H., Drieu L., Moulonguet-Doleris L., Ardaillou R. Renal excretion of antidiuretic hormone in healthy subjects and patients with renal failure. Clin Sci (Lond) 1984 Sep;67(3):307–312. doi: 10.1042/cs0670307. [DOI] [PubMed] [Google Scholar]
- Sands J. M., Nonoguchi H., Knepper M. A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am J Physiol. 1987 Nov;253(5 Pt 2):F823–F832. doi: 10.1152/ajprenal.1987.253.5.F823. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Pang P. K., Seto J., McEnroe M., Lammek B., Manning M. Vasopressin analogs that antagonize antidiuretic responses by rats to the antidiuretic hormone. Science. 1981 Apr 3;212(4490):49–51. doi: 10.1126/science.7209515. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I., Strasheim A. AVP stimulates adenylyl cyclase and phospholipase C in reciprocal fashion in cultured RIMCT cells. Am J Physiol. 1990 Oct;259(4 Pt 1):C693–C696. doi: 10.1152/ajpcell.1990.259.4.C693. [DOI] [PubMed] [Google Scholar]
- Teitelbaum I. Vasopressin-stimulated phosphoinositide hydrolysis in cultured rat inner medullary collecting duct cells is mediated by the oxytocin receptor. J Clin Invest. 1991 Jun;87(6):2122–2126. doi: 10.1172/JCI115243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K., Nonoguchi H., Marumo F. Effects of endothelin on peptide-dependent cyclic adenosine monophosphate accumulation along the nephron segments of the rat. J Clin Invest. 1990 Jun;85(6):2014–2018. doi: 10.1172/JCI114667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura Y., Ogawa H., Chihara T., Kondo K., Onogawa T., Nakamura S., Mori T., Tominaga M., Yabuuchi Y. OPC-21268, an orally effective, nonpeptide vasopressin V1 receptor antagonist. Science. 1991 Apr 26;252(5005):572–574. doi: 10.1126/science.1850553. [DOI] [PubMed] [Google Scholar]
- Yamamura Y., Ogawa H., Yamashita H., Chihara T., Miyamoto H., Nakamura S., Onogawa T., Yamashita T., Hosokawa T., Mori T. Characterization of a novel aquaretic agent, OPC-31260, as an orally effective, nonpeptide vasopressin V2 receptor antagonist. Br J Pharmacol. 1992 Apr;105(4):787–791. doi: 10.1111/j.1476-5381.1992.tb09058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]