Abstract
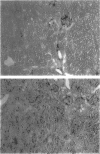
Proliferation and dedifferentiation of tubular cells are the hallmark of early regeneration after renal ischemic injury. Vimentin, a class III intermediate filament expressed only in mesenchymal cells of mature mammals, was shown to be transiently expressed in post-ischemic renal tubular epithelial cells. Vimentin re-expression was interpreted as a marker of cellular dedifferentiation, but its role in tubular regeneration after renal ischemia has also been hypothesized. This role was evaluated in mice bearing a null mutation of the vimentin gene. Expression of vimentin, proliferating cell nuclear antigen (a marker of cellular proliferation), and villin (a marker of differentiated brush-border membranes) was studied in wild-type (Vim+/+), heterozygous (Vim+/-), and homozygous (Vim-/-) mice subjected to transient ischemia of the left kidney. As expected, vimentin was detected by immunohistochemistry at the basal pole of proximal tubular cells from post-ischemic kidney in Vim+/+ and Vim+/- mice from day 2 to day 28. The expression of the reporter gene beta-galactosidase in Vim+/- and Vim-/- mice confirmed the tubular origin of vimentin. No compensatory expression of keratin could be demonstrated in Vim-/- mice. The intensity of proliferating cell nuclear antigen labeling and the pattern of villin expression were comparable in Vim-/-, Vim+/- and Vim+/+ mice at any time of the study. After 60 days, the structure of post-ischemic kidneys in Vim-/- mice was indistinguishable from that of normal non-operated kidneys in Vim+/+ mice. In conclusion, 1) the pattern of post-ischemic proximal tubular cell proliferation, differentiation, and tubular organization was not impaired in mice lacking vimentin and 2) these results suggest that the transient tubular expression of vimentin is not instrumental in tubular regeneration after renal ischemic injury.
Full text
PDF





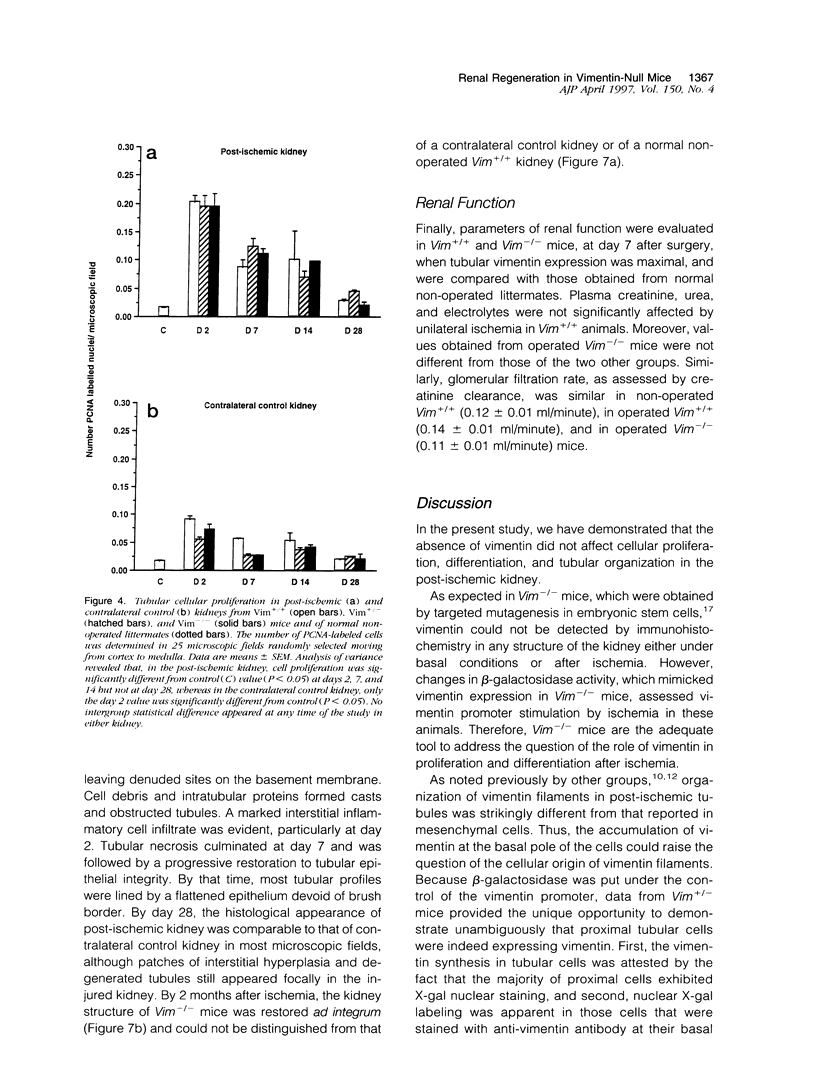

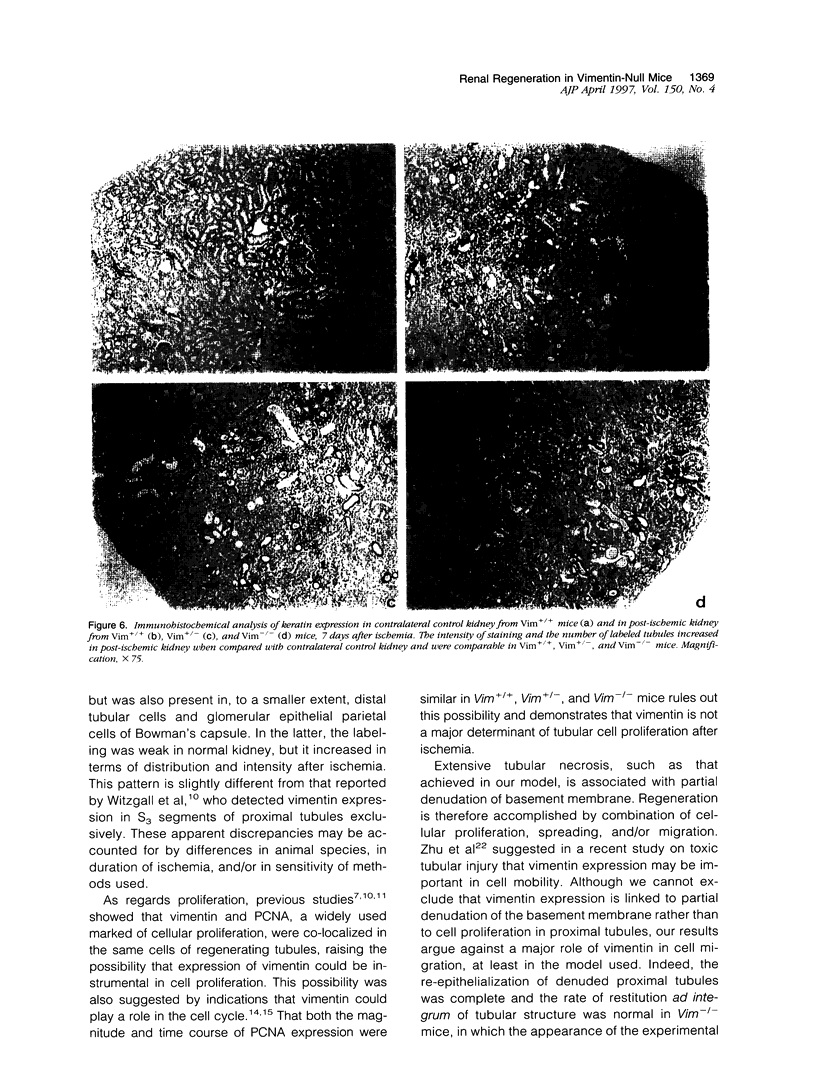


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. E., Gown A. M. Distribution and pattern of expression of villin, a gastrointestinal-associated cytoskeletal protein, in human carcinomas: a study employing paraffin-embedded tissue. Lab Invest. 1991 Mar;64(3):418–424. [PubMed] [Google Scholar]
- Bachmann S., Kriz W., Kuhn C., Franke W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry. 1983;77(3):365–394. doi: 10.1007/BF00490899. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E., Portier M. M., Dunia I., Paulin D., Pournin S., Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994 Nov 18;79(4):679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Dierick A. M., Praet M., Roels H., Verbeeck P., Robyns C., Oosterlinck W. Vimentin expression of renal cell carcinoma in relation to DNA content and histological grading: a combined light microscopic, immunocytochemical and cytophotometrical analysis. Histopathology. 1991 Apr;18(4):315–322. doi: 10.1111/j.1365-2559.1991.tb00852.x. [DOI] [PubMed] [Google Scholar]
- GOSS R. J. KINETICS OF COMPENSATORY GROWTH. Q Rev Biol. 1965 Jun;40:123–146. doi: 10.1086/404538. [DOI] [PubMed] [Google Scholar]
- Gossrau R., Günther T., Graf R. Enhancement of gentamicin-induced nephrotoxicity by Mg deficiency in non-pregnant rats. Morphological, enzyme histochemical and immunohistochemical studies. Histochemistry. 1989;90(6):489–496. doi: 10.1007/BF00494361. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Lehto V. P., Lehtonen E., Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984 May;50(5):552–559. [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments: new proteins, some answers, more questions. Curr Opin Cell Biol. 1995 Feb;7(1):46–54. doi: 10.1016/0955-0674(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Maunoury R., Robine S., Pringault E., Huet C., Guénet J. L., Gaillard J. A., Louvard D. Villin expression in the visceral endoderm and in the gut anlage during early mouse embryogenesis. EMBO J. 1988 Nov;7(11):3321–3329. doi: 10.1002/j.1460-2075.1988.tb03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunoury R., Robine S., Pringault E., Léonard N., Gaillard J. A., Louvard D. Developmental regulation of villin gene expression in the epithelial cell lineages of mouse digestive and urogenital tracts. Development. 1992 Jul;115(3):717–728. doi: 10.1242/dev.115.3.717. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Falk S. A., Dahl R. H. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest. 1989 Oct;84(4):1334–1339. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouwen E. J., Verstrepen W. A., Buyssens N., Zhu M. Q., De Broe M. E. Hyperplasia, hypertrophy, and phenotypic alterations in the distal nephron after acute proximal tubular injury in the rat. Lab Invest. 1994 Apr;70(4):479–493. [PubMed] [Google Scholar]
- Rittling S. R., Baserga R. Functional analysis and growth factor regulation of the human vimentin promoter. Mol Cell Biol. 1987 Nov;7(11):3908–3915. doi: 10.1128/mcb.7.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Spiegel D. M., Wilson P. D., Molitoris B. A. Epithelial polarity following ischemia: a requirement for normal cell function. Am J Physiol. 1989 Mar;256(3 Pt 2):F430–F436. doi: 10.1152/ajprenal.1989.256.3.F430. [DOI] [PubMed] [Google Scholar]
- Toubeau G., Nonclercq D., Zanen J., Laurent G., Schaudies P. R., Heuson-Stiennon J. A. Renal tissue expression of EGF and EGF receptor after ischaemic tubular injury: an immunohistochemical study. Exp Nephrol. 1994 Jul-Aug;2(4):229–239. [PubMed] [Google Scholar]
- Traub P., Shoeman R. L. Intermediate filament and related proteins: potential activators of nucleosomes during transcription initiation and elongation? Bioessays. 1994 May;16(5):349–355. doi: 10.1002/bies.950160510. [DOI] [PubMed] [Google Scholar]
- Waldherr R., Schwechheimer K. Co-expression of cytokeratin and vimentin intermediate-sized filaments in renal cell carcinomas. Comparative study of the intermediate-sized filament distribution in renal cell carcinomas and normal human kidney. Virchows Arch A Pathol Anat Histopathol. 1985;408(1):15–27. doi: 10.1007/BF00739959. [DOI] [PubMed] [Google Scholar]
- Wallin A., Zhang G., Jones T. W., Jaken S., Stevens J. L. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992 Apr;66(4):474–484. [PubMed] [Google Scholar]
- Ward J. M., Stevens J. L., Konishi N., Kurata Y., Uno H., Diwan B. A., Ohmori T. Vimentin metaplasia in renal cortical tubules of preneoplastic, neoplastic, aging, and regenerative lesions of rats and humans. Am J Pathol. 1992 Oct;141(4):955–964. [PMC free article] [PubMed] [Google Scholar]
- Witzgall R., Brown D., Schwarz C., Bonventre J. V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994 May;93(5):2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]