Abstract
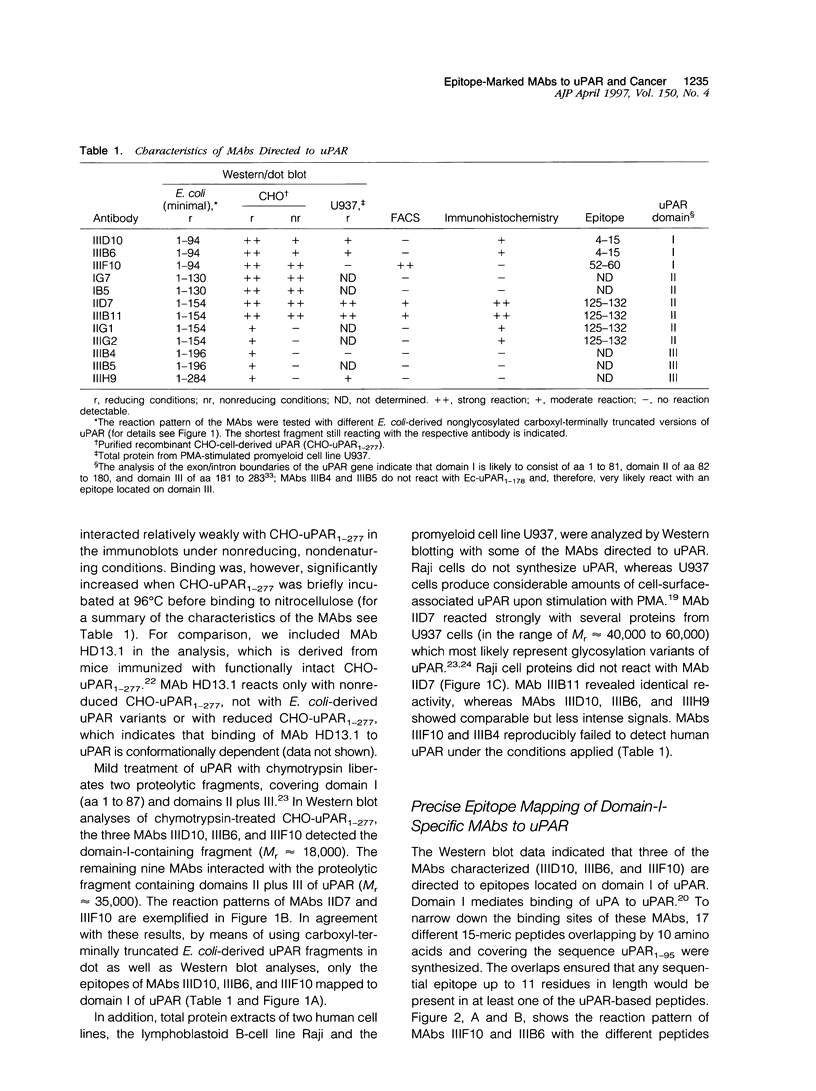
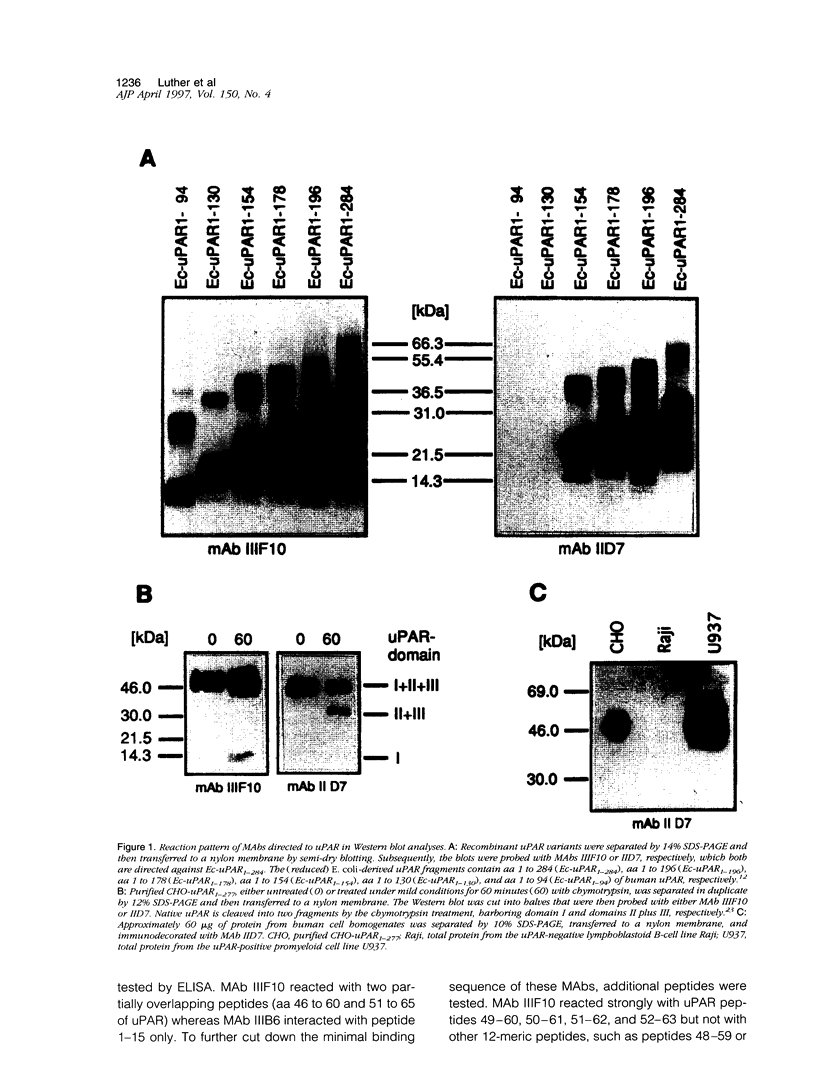
uPAR (CD87), the receptor for the urokinase-type plasminogen activator (uPA) facilitates tumor cell invasion and metastasis by focusing uPA proteolytic activity to the cell surface. As uPAR exists in various molecular forms, it is desirable to use well defined antibodies for analyses of uPAR antigen expression in human malignant tumors by immunological methods. Therefore, twelve monoclonal antibodies (MAbs) directed against uPAR were generated by using nonglycosylated, recombinant human uPAR (spanning amino acids 1 to 284), expressed in Escherichia coli, as the immunogen. The reaction pattern of these MAbs with the immunogen and a series of carboxyl-terminally truncated versions of uPAR demonstrated that at least six different epitopes of uPAR are recognized. All MAbs reacted under reducing conditions in immunoblot analyses with E. coli-expressed uPA and also with highly glycosylated, functionally intact, recombinant human uPAR expressed in Chinese hamster ovary (CHO) cells. Seven of the MAbs recognized CHO uPAR under nonreducing conditions as well. By flow cytofluorometric analyses, three of these MAbs were shown to bind to native human uPAR present on the cell surface of monocytoid U937 cells with MAb IIIF10 being the best. Saturation of uPAR with uPA on U937 cells completely blocked interaction of MAb IIIF10 with uPAR (mapped epitope, amino acids 52 to 60 of domain I of uPAR). In turn, preincubation of U937 cells with MAb IIIF10 efficiently reduced binding of uPA to uPAR, indicating that the epitope detected by MAb IIIF10 is located within or closely to the uPA-binding site of uPAR, and thus, this site may be a target to influence uPA/uPAR-mediated proteolysis in tumors. Binding of MAbs IID7 or IIIB11 (mapped epitope, amino acids 125 to 132 of domain II of uPAR) to uPAR is not affected when uPAR is occupied by uPA. As these MAbs reacted strongly with cellular uPAR antigen in formalin-fixed paraffin-embedded tumor sections, the domain-II-specific antibodies IID7 and IIIB11 may be useful for immunohistochemical studies of uPAR expression in tissue remodeling processes in tumor invasion. In conclusion, we have devised well defined and epitope-mapped MAbs to uPAR that are highly specific tools for detection and targeting of uPAR in tumor tissue.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S., Luther T., Grossmann H., Flössel C., Kotzsch M., Müller M. An ELISA for tissue factor using monoclonal antibodies. Blood Coagul Fibrinolysis. 1992 Jun;3(3):263–270. doi: 10.1097/00001721-199206000-00005. [DOI] [PubMed] [Google Scholar]
- Andreasen P. A., Sottrup-Jensen L., Kjøller L., Nykjaer A., Moestrup S. K., Petersen C. M., Gliemann J. Receptor-mediated endocytosis of plasminogen activators and activator/inhibitor complexes. FEBS Lett. 1994 Feb 7;338(3):239–245. doi: 10.1016/0014-5793(94)80276-9. [DOI] [PubMed] [Google Scholar]
- Barlos K., Chatzi O., Gatos D., Stavropoulos G. 2-Chlorotrityl chloride resin. Studies on anchoring of Fmoc-amino acids and peptide cleavage. Int J Pept Protein Res. 1991 Jun;37(6):513–520. [PubMed] [Google Scholar]
- Behrendt N., Ploug M., Patthy L., Houen G., Blasi F., Danø K. The ligand-binding domain of the cell surface receptor for urokinase-type plasminogen activator. J Biol Chem. 1991 Apr 25;266(12):7842–7847. [PubMed] [Google Scholar]
- Behrendt N., Rønne E., Ploug M., Petri T., Løber D., Nielsen L. S., Schleuning W. D., Blasi F., Appella E., Danø K. The human receptor for urokinase plasminogen activator. NH2-terminal amino acid sequence and glycosylation variants. J Biol Chem. 1990 Apr 15;265(11):6453–6460. [PubMed] [Google Scholar]
- Bianchi E., Cohen R. L., Thor A. T., Todd R. F., 3rd, Mizukami I. F., Lawrence D. A., Ljung B. M., Shuman M. A., Smith H. S. The urokinase receptor is expressed in invasive breast cancer but not in normal breast tissue. Cancer Res. 1994 Feb 15;54(4):861–866. [PubMed] [Google Scholar]
- Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. Bioessays. 1993 Feb;15(2):105–111. doi: 10.1002/bies.950150206. [DOI] [PubMed] [Google Scholar]
- Carriero M. V., Franco P., Del Vecchio S., Massa O., Botti G., D'Aiuto G., Stoppelli M. P., Salvatore M. Tissue distribution of soluble and receptor-bound urokinase in human breast cancer using a panel of monoclonal antibodies. Cancer Res. 1994 Oct 15;54(20):5445–5454. [PubMed] [Google Scholar]
- Casey J. R., Petranka J. G., Kottra J., Fleenor D. E., Rosse W. F. The structure of the urokinase-type plasminogen activator receptor gene. Blood. 1994 Aug 15;84(4):1151–1156. [PubMed] [Google Scholar]
- Duggan C., Maguire T., McDermott E., O'Higgins N., Fennelly J. J., Duffy M. J. Urokinase plasminogen activator and urokinase plasminogen activator receptor in breast cancer. Int J Cancer. 1995 May 29;61(5):597–600. doi: 10.1002/ijc.2910610502. [DOI] [PubMed] [Google Scholar]
- Fazioli F., Blasi F. Urokinase-type plasminogen activator and its receptor: new targets for anti-metastatic therapy? Trends Pharmacol Sci. 1994 Jan;15(1):25–29. doi: 10.1016/0165-6147(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Fields G. B., Noble R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990 Mar;35(3):161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Flössel C., Luther T., Müller M., Albrecht S., Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry. 1994 Jul;101(6):449–453. doi: 10.1007/BF00269495. [DOI] [PubMed] [Google Scholar]
- Høyer-Hansen G., Rønne E., Solberg H., Behrendt N., Ploug M., Lund L. R., Ellis V., Danø K. Urokinase plasminogen activator cleaves its cell surface receptor releasing the ligand-binding domain. J Biol Chem. 1992 Sep 5;267(25):18224–18229. [PubMed] [Google Scholar]
- Kanse S. M., Kost C., Wilhelm O. G., Andreasen P. A., Preissner K. T. The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp Cell Res. 1996 May 1;224(2):344–353. doi: 10.1006/excr.1996.0144. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995 May 1;55(9):1856–1862. [PubMed] [Google Scholar]
- Konrad M., Merz W. E. Regulation of N-glycosylation. Long term effect of cyclic AMP mediates enhanced synthesis of the dolichol pyrophosphate core oligosaccharide. J Biol Chem. 1994 Mar 25;269(12):8659–8666. [PubMed] [Google Scholar]
- Lau H. K., Kim M., Koo J., Chiu B., Murray D. Increase of a urokinase receptor-related low-molecular-weight molecule in colorectal adenocarcinomas. Clin Exp Metastasis. 1995 Nov;13(6):492–498. doi: 10.1007/BF00118188. [DOI] [PubMed] [Google Scholar]
- Liu G., Shuman M. A., Cohen R. L. Co-expression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung cancer cells. Int J Cancer. 1995 Feb 8;60(4):501–506. doi: 10.1002/ijc.2910600413. [DOI] [PubMed] [Google Scholar]
- Luther T., Flössel C., Albrecht S., Kotzsch M., Müller M. Tissue factor expression in normal and abnormal mammary gland. Nat Med. 1996 May;2(5):491–492. doi: 10.1038/nm0596-491a. [DOI] [PubMed] [Google Scholar]
- Luther T., Flössel C., Mackman N., Bierhaus A., Kasper M., Albrecht S., Sage E. H., Iruela-Arispe L., Grossmann H., Ströhlein A. Tissue factor expression during human and mouse development. Am J Pathol. 1996 Jul;149(1):101–113. [PMC free article] [PubMed] [Google Scholar]
- Magdolen V., Rettenberger P., Koppitz M., Goretzki L., Kessler H., Weidle U. H., König B., Graeff H., Schmitt M., Wilhelm O. Systematic mutational analysis of the receptor-binding region of the human urokinase-type plasminogen activator. Eur J Biochem. 1996 May 1;237(3):743–751. doi: 10.1111/j.1432-1033.1996.0743p.x. [DOI] [PubMed] [Google Scholar]
- Magdolen V., Rettenberger P., Lopens A., Oi H., Lottspeich F., Kellermann J., Creutzburg S., Goretzki L., Weidle U. H., Wilhelm O. Expression of the human urokinase-type plasminogen activator receptor in E. coli and Chinese hamster ovary cells: purification of the recombinant proteins and generation of polyclonal antibodies in chicken. Electrophoresis. 1995 May;16(5):813–816. doi: 10.1002/elps.11501601134. [DOI] [PubMed] [Google Scholar]
- Mizukami I. F., Garni-Wagner B. A., DeAngelo L. M., Liebert M., Flint A., Lawrence D. A., Cohen R. L., Todd R. F., 3rd Immunologic detection of the cellular receptor for urokinase plasminogen activator. Clin Immunol Immunopathol. 1994 Apr;71(1):96–104. doi: 10.1006/clin.1994.1057. [DOI] [PubMed] [Google Scholar]
- Møller L. B., Pöllänen J., Rønne E., Pedersen N., Blasi F. N-linked glycosylation of the ligand-binding domain of the human urokinase receptor contributes to the affinity for its ligand. J Biol Chem. 1993 May 25;268(15):11152–11159. [PubMed] [Google Scholar]
- Pedersen N., Schmitt M., Rønne E., Nicoletti M. I., Høyer-Hansen G., Conese M., Giavazzi R., Dano K., Kuhn W., Jänicke F. A ligand-free, soluble urokinase receptor is present in the ascitic fluid from patients with ovarian cancer. J Clin Invest. 1993 Nov;92(5):2160–2167. doi: 10.1172/JCI116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picone R., Kajtaniak E. L., Nielsen L. S., Behrendt N., Mastronicola M. R., Cubellis M. V., Stoppelli M. P., Pedersen S., Danø K., Blasi F. Regulation of urokinase receptors in monocytelike U937 cells by phorbol ester phorbol myristate acetate. J Cell Biol. 1989 Feb;108(2):693–702. doi: 10.1083/jcb.108.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug M., Rahbek-Nielsen H., Ellis V., Roepstorff P., Danø K. Chemical modification of the urokinase-type plasminogen activator and its receptor using tetranitromethane. Evidence for the involvement of specific tyrosine residues in both molecules during receptor-ligand interaction. Biochemistry. 1995 Oct 3;34(39):12524–12534. doi: 10.1021/bi00039a006. [DOI] [PubMed] [Google Scholar]
- Ploug M., Rønne E., Behrendt N., Jensen A. L., Blasi F., Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991 Jan 25;266(3):1926–1933. [PubMed] [Google Scholar]
- Pyke C., Eriksen J., Solberg H., Nielsen B. S., Kristensen P., Lund L. R., Danø K. An alternatively spliced variant of mRNA for the human receptor for urokinase plasminogen activator. FEBS Lett. 1993 Jul 12;326(1-3):69–74. doi: 10.1016/0014-5793(93)81763-p. [DOI] [PubMed] [Google Scholar]
- Pyke C., Graem N., Ralfkiaer E., Rønne E., Høyer-Hansen G., Brünner N., Danø K. Receptor for urokinase is present in tumor-associated macrophages in ductal breast carcinoma. Cancer Res. 1993 Apr 15;53(8):1911–1915. [PubMed] [Google Scholar]
- Pyke C., Ralfkiaer E., Rønne E., Høyer-Hansen G., Kirkeby L., Danø K. Immunohistochemical detection of the receptor for urokinase plasminogen activator in human colon cancer. Histopathology. 1994 Feb;24(2):131–138. doi: 10.1111/j.1365-2559.1994.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Pöllänen J. J. The N-terminal domain of human urokinase receptor contains two distinct regions critical for ligand recognition. Blood. 1993 Nov 1;82(9):2719–2729. [PubMed] [Google Scholar]
- Rettenberger P., Wilhelm O., Oi H., Weidle U. H., Goretzki L., Koppitz M., Lottspeich F., König B., Pessara U., Kramer M. D. A competitive chromogenic assay to study the functional interaction of urokinase-type plasminogen activator with its receptor. Biol Chem Hoppe Seyler. 1995 Oct;376(10):587–594. doi: 10.1515/bchm3.1995.376.10.587. [DOI] [PubMed] [Google Scholar]
- Rønne E., Behrendt N., Ellis V., Ploug M., Danø K., Høyer-Hansen G. Cell-induced potentiation of the plasminogen activation system is abolished by a monoclonal antibody that recognizes the NH2-terminal domain of the urokinase receptor. FEBS Lett. 1991 Aug 19;288(1-2):233–236. doi: 10.1016/0014-5793(91)81042-7. [DOI] [PubMed] [Google Scholar]
- Schmitt M., Wilhelm O., Jänicke F., Magdolen V., Reuning U., Ohi H., Moniwa N., Kobayashi H., Weidle U., Graeff H. Urokinase-type plasminogen activator (uPA) and its receptor (CD87): a new target in tumor invasion and metastasis. J Obstet Gynaecol (Tokyo 1995) 1995 Apr;21(2):151–165. doi: 10.1111/j.1447-0756.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Solberg H., Rømer J., Brünner N., Holm A., Sidenius N., Danø K., Høyer-Hansen G. A cleaved form of the receptor for urokinase-type plasminogen activator in invasive transplanted human and murine tumors. Int J Cancer. 1994 Sep 15;58(6):877–881. doi: 10.1002/ijc.2910580622. [DOI] [PubMed] [Google Scholar]
- Wagner S. N., Atkinson M. J., Thanner S., Wagner C., Schmitt M., Wilhelm O., Rotter M., Höfler H. Modulation of urokinase and urokinase receptor gene expression in human renal cell carcinoma. Am J Pathol. 1995 Jul;147(1):183–192. [PMC free article] [PubMed] [Google Scholar]
- Wilhelm O., Weidle U., Höhl S., Rettenberger P., Schmitt M., Graeff H. Recombinant soluble urokinase receptor as a scavenger for urokinase-type plasminogen activator (uPA). Inhibition of proliferation and invasion of human ovarian cancer cells. FEBS Lett. 1994 Jan 10;337(2):131–134. doi: 10.1016/0014-5793(94)80259-9. [DOI] [PubMed] [Google Scholar]
- Xing R. H., Rabbani S. A. Overexpression of urokinase receptor in breast cancer cells results in increased tumor invasion, growth and metastasis. Int J Cancer. 1996 Jul 29;67(3):423–429. doi: 10.1002/(SICI)1097-0215(19960729)67:3<423::AID-IJC18>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]