Abstract
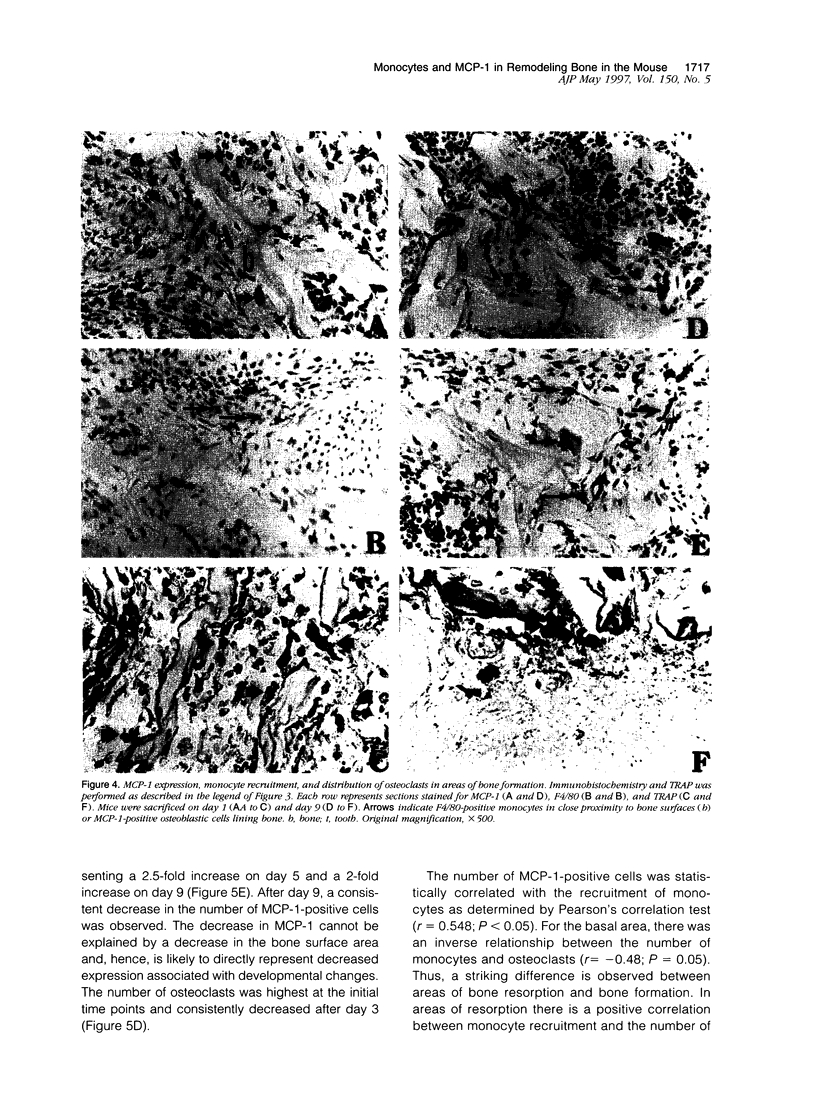
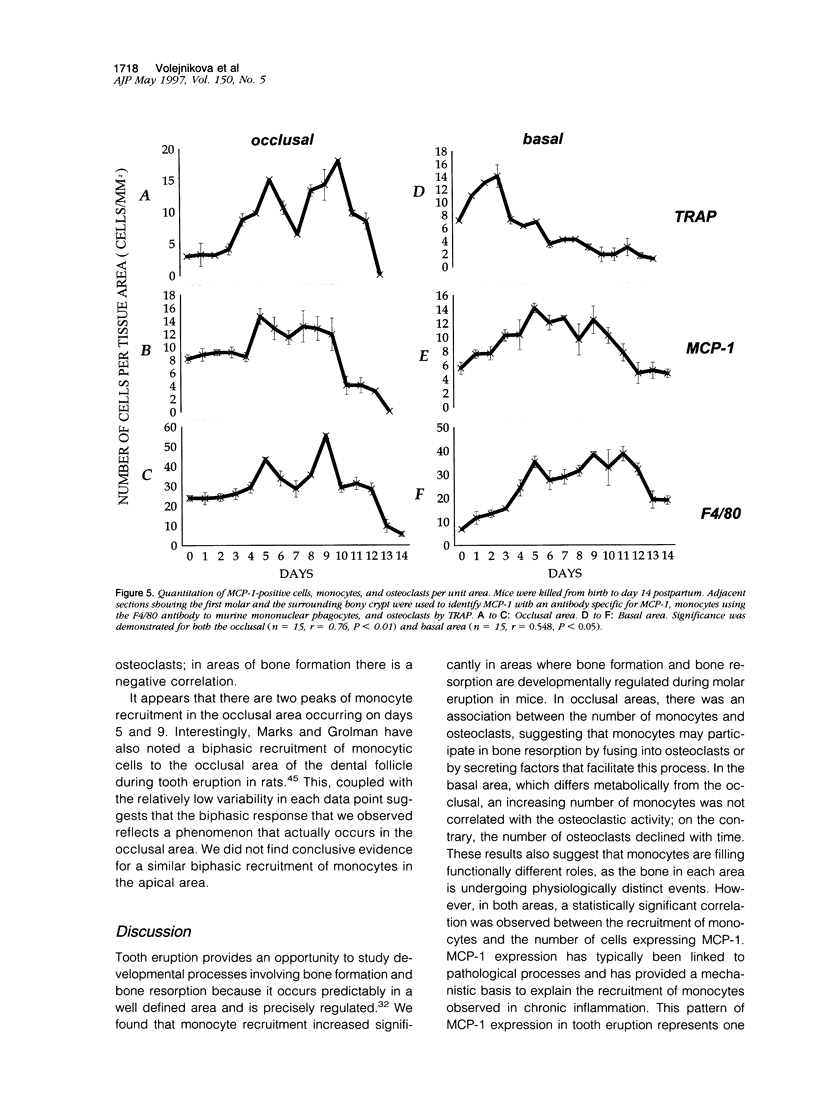
Tooth eruption is defined as the movement of a tooth from its site of development within the alveolar bone to its position of function in the oral cavity. It represents an excellent model to examine osseous metabolism as bone resorption and bone formation occur simultaneously and are spatially separated. Bone resorption occurs in the coronal (occlusal) area, whereas bone formation occurs in the basal area. Monocytes are thought to have a significant role in the regulation of osseous metabolism. The goal of this study was to examine the recruitment of monocytes to bone in C57BL/6J mice that are undergoing developmentally regulated bone remodeling. Monocytes were detected by immunohistochemistry and osteoclasts were counted as bone-associated multi-nucleated, tartrate-resistant acid phosphatase (TRAP)-positive cells. Cell numbers were obtained from histological sections of animals sacrificed daily for 14 days after birth; an image analysis system was used for quantification. The results demonstrated that, immediately after birth, there were relatively few monocytic cells. In the area of bone resorption, the number of monocytes increased with time, reaching peaks at 5 and 9 days, and decreased thereafter. A similar pattern was observed for osteoclasts. In the area of bone formation, there was a time-dependent increase in the number of monocytes. In contrast, the number of osteoclasts in this area was highest at the earliest time points and decreased after day 3. To investigate potential mechanisms for the recruitment of monocytes, expression of monocyte chemoattractant protein (MCP)-1 was assessed. The number of MCP-1-positive cells increased with time and was generally proportional to the recruitment of mononuclear phagocytes. Osteoblasts were the principal bone cell type expressing MCP-1. The results demonstrate that the recruitment of mononuclear cells in the occlusal area is associated with bone resorption. In contrast, recruitment of monocytes in the basal area is associated with bone formation and a decrease in the number of osteoclasts. These results suggest that monocytes have different functional roles in areas of bone formation compared with bone resorption. Furthermore, the expression of MCP-1 is developmentally regulated and may provide a mechanistic basis to explain the recruitment of monocytic cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Neville-Golden J., Galanopoulos T., Kradin R. L., Valente A. J., Graves D. T. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5371–5375. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Dahinden C. A. CC chemokines in allergic inflammation. Immunol Today. 1994 Mar;15(3):127–133. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Bolander M. E. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992 Jun;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Burger E. H., van der Meer J. W., Nijweide P. J. Osteoclast formation from mononuclear phagocytes: role of bone-forming cells. J Cell Biol. 1984 Dec;99(6):1901–1906. doi: 10.1083/jcb.99.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill D. R., Marks S. C., Jr Chronology and histology of exfoliation and eruption of mandibular premolars in dogs. J Morphol. 1982 Feb;171(2):213–218. doi: 10.1002/jmor.1051710208. [DOI] [PubMed] [Google Scholar]
- Cahill D. R., Marks S. C., Jr Tooth eruption: evidence for the central role of the dental follicle. J Oral Pathol. 1980 Jul;9(4):189–200. doi: 10.1111/j.1600-0714.1980.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Cuadros M. A., Martin C., Coltey P., Almendros A., Navascués J. First appearance, distribution, and origin of macrophages in the early development of the avian central nervous system. J Comp Neurol. 1993 Apr 1;330(1):113–129. doi: 10.1002/cne.903300110. [DOI] [PubMed] [Google Scholar]
- Gordon S., Lawson L., Rabinowitz S., Crocker P. R., Morris L., Perry V. H. Antigen markers of macrophage differentiation in murine tissues. Curr Top Microbiol Immunol. 1992;181:1–37. doi: 10.1007/978-3-642-77377-8_1. [DOI] [PubMed] [Google Scholar]
- Grandaliano G., Valente A. J., Rozek M. M., Abboud H. E. Gamma interferon stimulates monocyte chemotactic protein (MCP-1) in human mesangial cells. J Lab Clin Med. 1994 Feb;123(2):282–289. [PubMed] [Google Scholar]
- Hanazawa S., Takeshita A., Amano S., Semba T., Nirazuka T., Katoh H., Kitano S. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 May 5;268(13):9526–9532. [PubMed] [Google Scholar]
- Hanazawa S., Takeshita A., Tsukamoto Y., Kawata Y., Takara K. O., Kitano S. Transforming growth factor-beta-induced gene expression of monocyte chemoattractant JE in mouse osteoblastic cells, MC3T3-E1. Biochem Biophys Res Commun. 1991 Oct 31;180(2):1130–1136. doi: 10.1016/s0006-291x(05)81184-2. [DOI] [PubMed] [Google Scholar]
- Hopkinson-Woolley J., Hughes D., Gordon S., Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994 May;107(Pt 5):1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- Hume D. A., Robinson A. P., MacPherson G. G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80. Relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983 Nov 1;158(5):1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka T., Cielinski M., Aukerman S. L., Marks S. C., Jr The effects of colony-stimulating factor-1 on tooth eruption in the toothless (osteopetrotic) rat in relation to the critical periods for bone resorption during tooth eruption. Arch Oral Biol. 1992 Aug;37(8):629–636. doi: 10.1016/0003-9969(92)90125-r. [DOI] [PubMed] [Google Scholar]
- Ip M. M., Shoemaker S. F., Darcy K. M. Regulation of rat mammary epithelial cell proliferation and differentiation by tumor necrosis factor-alpha. Endocrinology. 1992 May;130(5):2833–2844. doi: 10.1210/endo.130.5.1572296. [DOI] [PubMed] [Google Scholar]
- Jaskoll T., Boyer P. D., Melnick M. Tumor necrosis factor-alpha and embryonic mouse lung morphogenesis. Dev Dyn. 1994 Oct;201(2):137–150. doi: 10.1002/aja.1002010205. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T. Biological aspects of monocyte chemoattractant protein-1 (MCP-1). Adv Exp Med Biol. 1991;305:57–64. doi: 10.1007/978-1-4684-6009-4_7. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Cahill D. R., Wise G. E. The cytology of the dental follicle and adjacent alveolar bone during tooth eruption in the dog. Am J Anat. 1983 Nov;168(3):277–289. doi: 10.1002/aja.1001680303. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr, Gorski J. P., Wise G. E. The mechanisms and mediators of tooth eruption--models for developmental biologists. Int J Dev Biol. 1995 Feb;39(1):223–230. [PubMed] [Google Scholar]
- Marks S. C., Jr, Grolman M. L. Tartrate-resistant acid phosphatase in mononuclear and multinuclear cells during the bone resorption of tooth eruption. J Histochem Cytochem. 1987 Nov;35(11):1227–1230. doi: 10.1177/35.11.3655324. [DOI] [PubMed] [Google Scholar]
- Marks S. C., Jr Tooth eruption and bone resorption: experimental investigation of the ia (osteopetrotic) rat as a model for studying their relationships. J Oral Pathol. 1976 May;5(3):149–163. doi: 10.1111/j.1600-0714.1976.tb01760.x. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Minkin C. Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int. 1982 May;34(3):285–290. doi: 10.1007/BF02411252. [DOI] [PubMed] [Google Scholar]
- Morris L., Graham C. F., Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991 Jun;112(2):517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi P., Wang C. Y., Stashenko P., Lee S. K., Lorenzo J. A., Graves D. T. Monocyte chemoattractant protein-1 expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology. 1995 Jun;136(6):2752–2759. doi: 10.1210/endo.136.6.7750500. [DOI] [PubMed] [Google Scholar]
- Rollins B. J., Stier P., Ernst T., Wong G. G. The human homolog of the JE gene encodes a monocyte secretory protein. Mol Cell Biol. 1989 Nov;9(11):4687–4695. doi: 10.1128/mcb.9.11.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990 Jun;136(6):1229–1233. [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Dewhirst F. E., Peros W. J., Kent R. L., Ago J. M. Synergistic interactions between interleukin 1, tumor necrosis factor, and lymphotoxin in bone resorption. J Immunol. 1987 Mar 1;138(5):1464–1468. [PubMed] [Google Scholar]
- Takeshita A., Hanazawa S., Amano S., Matumoto T., Kitano S. IL-1 induces expression of monocyte chemoattractant JE in clonal mouse osteoblastic cell line MC3T3-E1. J Immunol. 1993 Feb 15;150(4):1554–1562. [PubMed] [Google Scholar]
- Tinkler S. M., Linder J. E., Williams D. M., Johnson N. W. Formation of osteoclasts from blood monocytes during 1 alpha-OH Vit D-stimulated bone resorption in mice. J Anat. 1981 Oct;133(Pt 3):389–396. [PMC free article] [PubMed] [Google Scholar]
- Villiger P. M., Terkeltaub R., Lotz M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J Immunol. 1992 Jul 15;149(2):722–727. [PubMed] [Google Scholar]
- Wise G. E., Fan W. Changes in the tartrate-resistant acid phosphatase cell population in dental follicles and bony crypts of rat molars during tooth eruption. J Dent Res. 1989 Feb;68(2):150–156. doi: 10.1177/00220345890680021001. [DOI] [PubMed] [Google Scholar]
- Wise G. E., Lin F. The molecular biology of initiation of tooth eruption. J Dent Res. 1995 Jan;74(1):303–306. doi: 10.1177/00220345950740010301. [DOI] [PubMed] [Google Scholar]
- Wise G. E., Marks S. C., Jr, Cahill D. R. Ultrastructural features of the dental follicle associated with formation of the tooth eruption pathway in the dog. J Oral Pathol. 1985 Jan;14(1):15–26. doi: 10.1111/j.1600-0714.1985.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Yu X., Antoniades H. N., Graves D. T. Expression of monocyte chemoattractant protein 1 in human inflamed gingival tissues. Infect Immun. 1993 Nov;61(11):4622–4628. doi: 10.1128/iai.61.11.4622-4628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Barnhill R. L., Graves D. T. Expression of monocyte chemoattractant protein-1 in delayed type hypersensitivity reactions in the skin. Lab Invest. 1994 Aug;71(2):226–235. [PubMed] [Google Scholar]
- Yu X., Dluz S., Graves D. T., Zhang L., Antoniades H. N., Hollander W., Prusty S., Valente A. J., Schwartz C. J., Sonenshein G. E. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6953–6957. doi: 10.1073/pnas.89.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. F., Valente A. J., Lorenzo J. A., Carnes D., Graves D. T. Expression of monocyte chemoattractant protein 1 in human osteoblastic cells stimulated by proinflammatory mediators. J Bone Miner Res. 1994 Jul;9(7):1123–1130. doi: 10.1002/jbmr.5650090721. [DOI] [PubMed] [Google Scholar]