Abstract
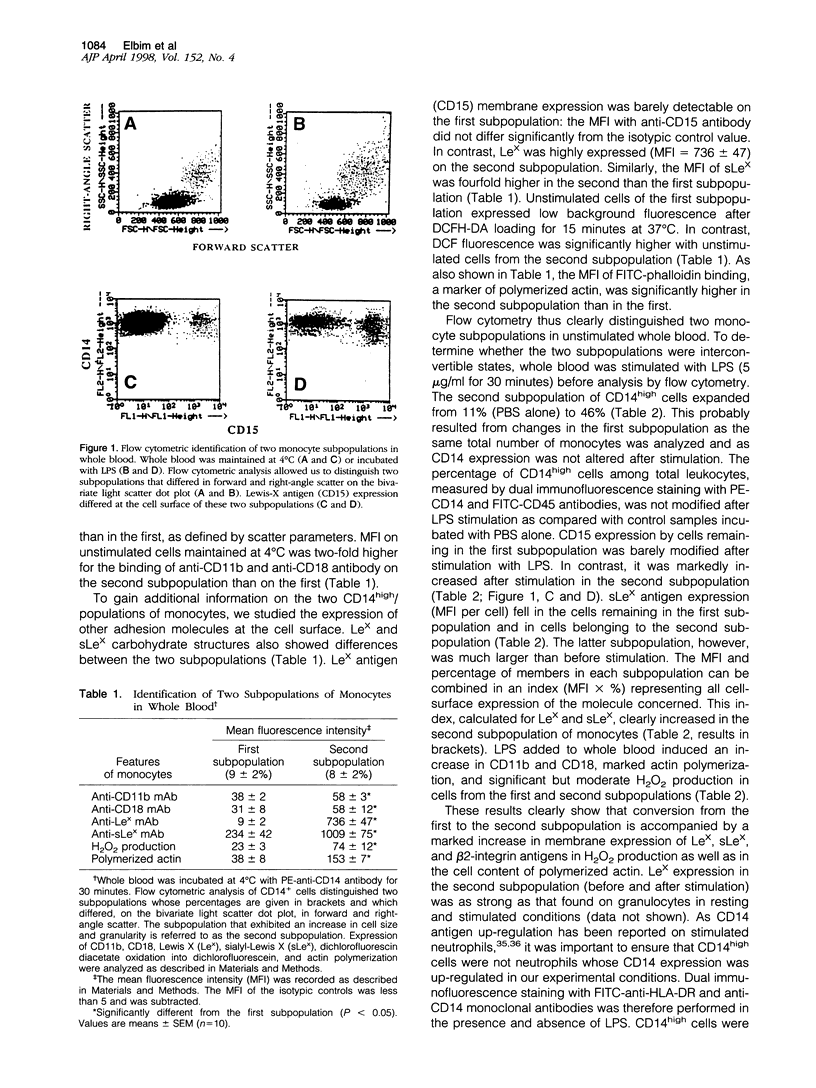
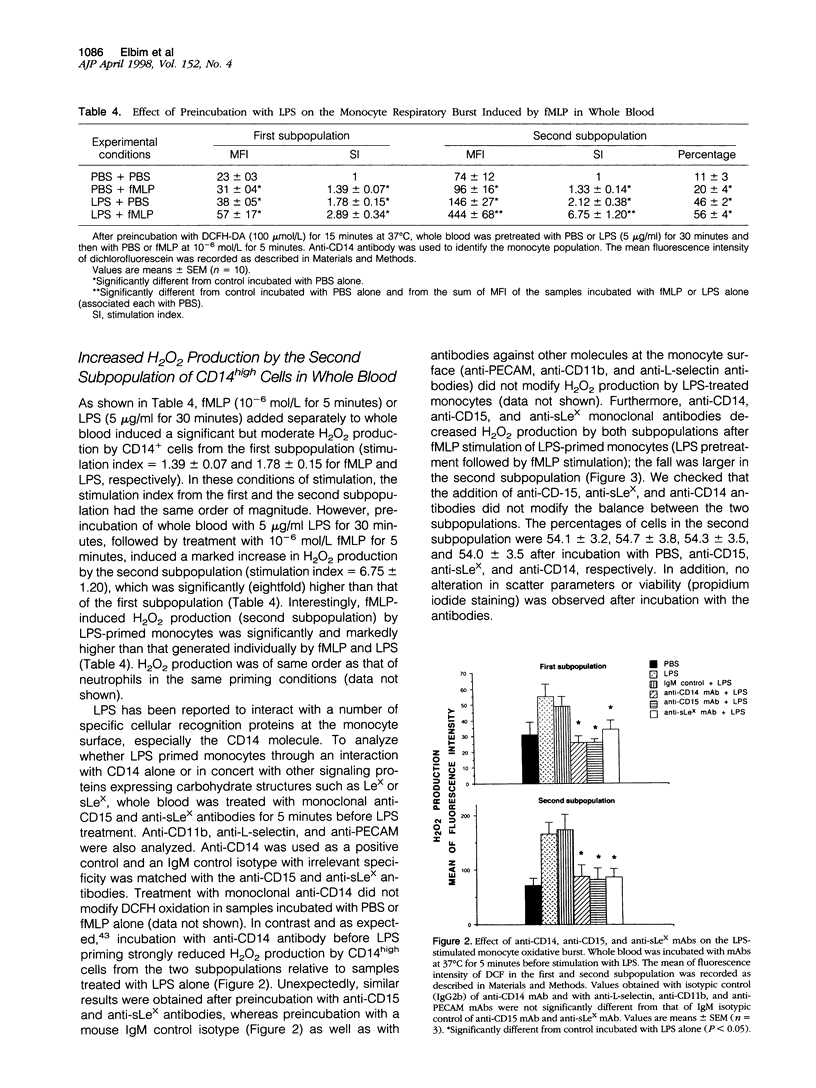
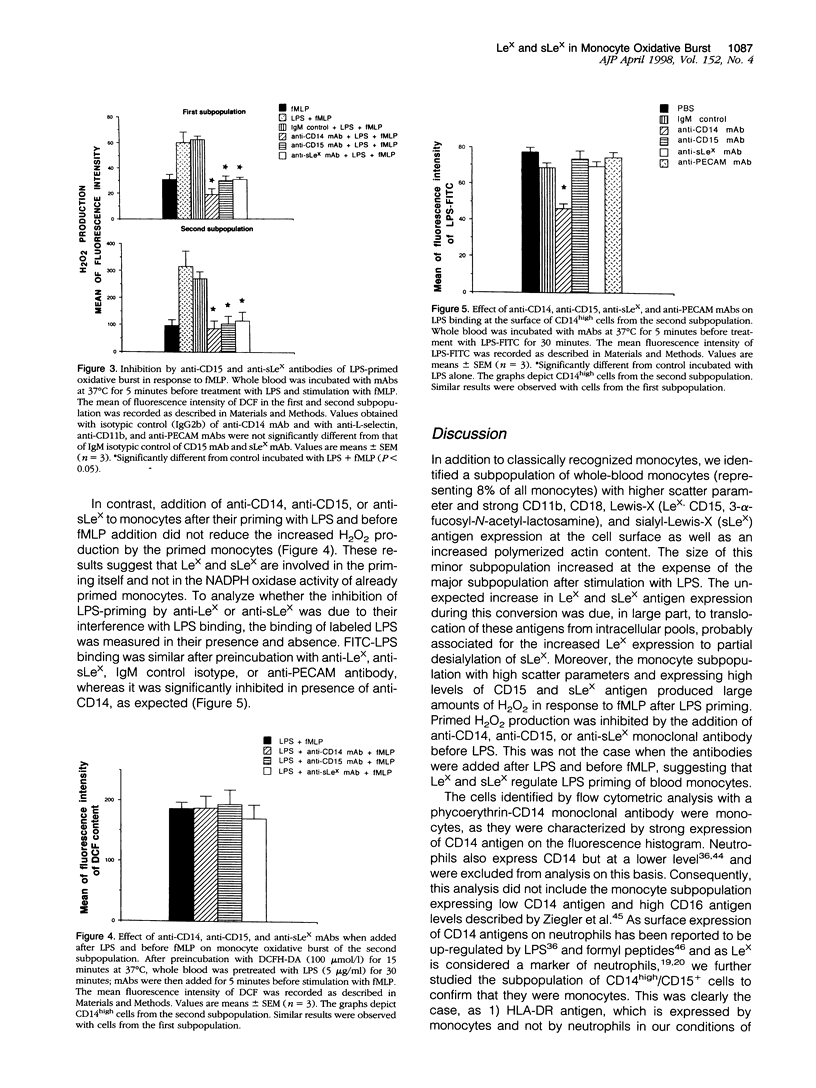
By using flow cytometric analysis of cells in whole blood expressing high levels of CD14, we found a subpopulation of monocytes (8% of total) with higher scatter parameters, high capacity to produce reactive oxygen species (ROS), stronger expression of Lewis-X (CD15), sialyl-Lewis-X, CD11b and CD18 antigens, as well as an increased polymerized actin content. The size of this subpopulation increased after stimulation with lipopolysaccharide at the expense of the remaining monocytes, suggesting that its features were inducible. The membrane increase in Lewis-X and sialyl-Lewis-X expression observed during this conversion was largely due to the translocation of these carbohydrate structures from intracellular pools. Moreover, this subpopulation behaved as a primed monocyte subpopulation producing large amounts of H2O2 in response to N-formyl-methionyl-leucyl-phenylalanine. Increased H2O2 production was inhibited not only by anti-CD14 but also by anti-CD15 and anti-sialyl-Lewis-X monoclonal antibodies when added before lipopolysaccharide. These results show that lipopolysaccharide priming is regulated, at least in part, by Lewis-X and sialyl-Lewis-X structures expressed on the monocyte membrane. All together, this highly reactive and inducible subpopulation of monocytes, which share phenotypic and functional characteristics with neutrophils, might play an important role in host defenses and inflammatory responses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar M., Amit N., Huu T. P., Chollet-Martin S., Labro M. T., Gougerot-Pocidalo M. A., Hakim J. Production by K 562 cells of an inhibitor of adherence-related functions of human neutrophils. J Immunol. 1990 Jun 15;144(12):4749–4756. [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arenson E. B., Jr, Epstein M. B., Seeger R. C. Volumetric and functional heterogeneity of human monocytes. J Clin Invest. 1980 Mar;65(3):613–618. doi: 10.1172/JCI109706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Bender J. G., McPhail L. C., Van Epps D. E. Exposure of human neutrophils to chemotactic factors potentiates activation of the respiratory burst enzyme. J Immunol. 1983 May;130(5):2316–2323. [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Mansson O., Butcher E. C., Magnani J. L. A carbohydrate domain common to both sialyl Le(a) and sialyl Le(X) is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J Biol Chem. 1991 Aug 15;266(23):14869–14872. [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier C., Jahns G., Kazatchkine M. D., Haeffner-Cavaillon N. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-alpha by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1992 Jun;22(6):1461–1466. doi: 10.1002/eji.1830220619. [DOI] [PubMed] [Google Scholar]
- Elbim C., Chollet-Martin S., Bailly S., Hakim J., Gougerot-Pocidalo M. A. Priming of polymorphonuclear neutrophils by tumor necrosis factor alpha in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993 Jul 15;82(2):633–640. [PubMed] [Google Scholar]
- Elbim C., Prevot M. H., Bouscarat F., Franzini E., Chollet-Martin S., Hakim J., Gougerot-Pocidalo M. A. Polymorphonuclear neutrophils from human immunodeficiency virus-infected patients show enhanced activation, diminished fMLP-induced L-selectin shedding, and an impaired oxidative burst after cytokine priming. Blood. 1994 Oct 15;84(8):2759–2766. [PubMed] [Google Scholar]
- Frankenberger M., Sternsdorf T., Pechumer H., Pforte A., Ziegler-Heitbrock H. W. Differential cytokine expression in human blood monocyte subpopulations: a polymerase chain reaction analysis. Blood. 1996 Jan 1;87(1):373–377. [PubMed] [Google Scholar]
- Fukuda M. N., Dell A., Tiller P. R., Varki A., Klock J. C., Fukuda M. Structure of a novel sialylated fucosyl lacto-N-norhexaosylceramide isolated from chronic myelogenous leukemia cells. J Biol Chem. 1986 Feb 15;261(5):2376–2383. [PubMed] [Google Scholar]
- Gosselin E. J., Wardwell K., Rigby W. F., Guyre P. M. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993 Aug 1;151(3):1482–1490. [PubMed] [Google Scholar]
- Hawkins D. Neutrophilic leukocytes in immunologic reactions in vitro: effect of cytochalasin B. J Immunol. 1973 Jan;110(1):294–296. [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Enhancement of immunologically induced granule exocytosis from neutrophils by cytochalasin B. J Immunol. 1973 Jan;110(1):290–293. [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Kouassi E., Hmama Z., Lina G., Vial J., Faure-Barba F., Normier G., Binz H., Revillard J. P. Activation of human monocyte chemiluminescence response by acylpoly(1,3)galactosides derived from Klebsiella pneumoniae. J Leukoc Biol. 1992 Nov;52(5):529–536. doi: 10.1002/jlb.52.5.529. [DOI] [PubMed] [Google Scholar]
- Lacal P. M., Barasoaín I., Sánchez A., García-Sancho J., Flores I., Mollinedo F. A monoclonal antibody that detects a specific human neutrophil antigen involved in phorbol myristate acetate- and formyl-methionyl-leucyl-phenylalanine-triggered respiratory burst. J Immunol. 1992 Jan 1;148(1):161–168. [PubMed] [Google Scholar]
- Lambré C. R., Greffard A., Gattegno L., Saffar L. Modifications of sialidase activity during the monocyte-macrophage differentiation in vitro. Immunol Lett. 1990 Jan;23(3):179–182. doi: 10.1016/0165-2478(90)90188-v. [DOI] [PubMed] [Google Scholar]
- Landmann R., Scherer F., Schumann R., Link S., Sansano S., Zimmerli W. LPS directly induces oxygen radical production in human monocytes via LPS binding protein and CD14. J Leukoc Biol. 1995 Mar;57(3):440–449. doi: 10.1002/jlb.57.3.440. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F., Olweus J., Aarli A., Bjerknes R. Signal transduction in human monocytes and granulocytes through the PI-linked antigen CD14. FEBS Lett. 1990 Oct 29;273(1-2):55–58. doi: 10.1016/0014-5793(90)81049-t. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F., Olweus J. Signal transduction in monocytes and granulocytes measured by multiparameter flow cytometry. Cytometry. 1992;13(7):693–702. doi: 10.1002/cyto.990130705. [DOI] [PubMed] [Google Scholar]
- MacIntyre E. A., Roberts P. J., Jones M., Van der Schoot C. E., Favalaro E. J., Tidman N., Linch D. C. Activation of human monocytes occurs on cross-linking monocytic antigens to an Fc receptor. J Immunol. 1989 Apr 1;142(7):2377–2383. [PubMed] [Google Scholar]
- Macey M. G., McCarthy D. A., Vordermeier S., Newland A. C., Brown K. A. Effects of cell purification methods on CD11b and L-selectin expression as well as the adherence and activation of leucocytes. J Immunol Methods. 1995 Apr 26;181(2):211–219. doi: 10.1016/0022-1759(95)00003-s. [DOI] [PubMed] [Google Scholar]
- Marchant A., Duchow J., Delville J. P., Goldman M. Lipopolysaccharide induces up-regulation of CD14 molecule on monocytes in human whole blood. Eur J Immunol. 1992 Jun;22(6):1663–1665. doi: 10.1002/eji.1830220650. [DOI] [PubMed] [Google Scholar]
- Melnick D. A., Meshulam T., Manto A., Malech H. L. Activation of human neutrophils by monoclonal antibody PMN7C3: cell movement and adhesion can be triggered independently from the respiratory burst. Blood. 1986 May;67(5):1388–1394. [PubMed] [Google Scholar]
- Nauseef W. M., Root R. K., Newman S. L., Malech H. L. Inhibition of zymosan activation of human neutrophil oxidative metabolism by a mouse monoclonal antibody. Blood. 1983 Sep;62(3):635–644. [PubMed] [Google Scholar]
- Nelson R. M., Dolich S., Aruffo A., Cecconi O., Bevilacqua M. P. Higher-affinity oligosaccharide ligands for E-selectin. J Clin Invest. 1993 Mar;91(3):1157–1166. doi: 10.1172/JCI116275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C. A., Campbell E. J., Stockley R. A. Monocyte adherence to fibronectin: role of CD11/CD18 integrins and relationship to other monocyte functions. J Leukoc Biol. 1992 Apr;51(4):400–408. doi: 10.1002/jlb.51.4.400. [DOI] [PubMed] [Google Scholar]
- Owen C. A., Campbell M. A., Boukedes S. S., Campbell E. J. Monocytes recruited to sites of inflammation express a distinctive proinflammatory (P) phenotype. Am J Physiol. 1994 Dec;267(6 Pt 1):L786–L796. doi: 10.1152/ajplung.1994.267.6.L786. [DOI] [PubMed] [Google Scholar]
- Owen C. A., Campbell M. A., Boukedes S. S., Stockley R. A., Campbell E. J. A discrete subpopulation of human monocytes expresses a neutrophil-like proinflammatory (P) phenotype. Am J Physiol. 1994 Dec;267(6 Pt 1):L775–L785. doi: 10.1152/ajplung.1994.267.6.L775. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Hamilton J. A. Priming of the respiratory burst of bone marrow-derived macrophages is associated with an increase in protein kinase C content. J Immunol. 1992 Aug 1;149(3):1016–1022. [PubMed] [Google Scholar]
- Shappell S. B., Toman C., Anderson D. C., Taylor A. A., Entman M. L., Smith C. W. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990 Apr 1;144(7):2702–2711. [PubMed] [Google Scholar]
- Skacel P. O., Edwards A. J., Harrison C. T., Watkins W. M. Enzymic control of the expression of the X determinant (CD15) in human myeloid cells during maturation: the regulatory role of 6-sialytransferase. Blood. 1991 Sep 15;78(6):1452–1460. [PubMed] [Google Scholar]
- Springer T. A., Lasky L. A. Cell adhesion. Sticky sugars for selectins. Nature. 1991 Jan 17;349(6306):196–197. doi: 10.1038/349196a0. [DOI] [PubMed] [Google Scholar]
- Stocks S. C., Albrechtsen M., Kerr M. A. Expression of the CD15 differentiation antigen (3-fucosyl-N-acetyl-lactosamine, LeX) on putative neutrophil adhesion molecules CR3 and NCA-160. Biochem J. 1990 Jun 1;268(2):275–280. doi: 10.1042/bj2680275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks S. C., Kerr M. A. Stimulation of neutrophil adhesion by antibodies recognizing CD15 (Le(X)) and CD15-expressing carcinoembryonic antigen-related glycoprotein NCA-160. Biochem J. 1992 Nov 15;288(Pt 1):23–27. doi: 10.1042/bj2880023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteroo P., Geurts van Kessel A. D. Expression of CD15 (FAL) on myeloid cells and chromosomal localization of the gene. Histochem J. 1992 Nov;24(11):777–782. doi: 10.1007/BF01046349. [DOI] [PubMed] [Google Scholar]
- Varki A. Selectin ligands. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell T. K., Fialkow L., Chan C. K., Kishimoto T. K., Downey G. P. Potentiation of the oxidative burst of human neutrophils. A signaling role for L-selectin. J Biol Chem. 1994 Jul 15;269(28):18485–18491. [PubMed] [Google Scholar]
- Weingarten R., Sklar L. A., Mathison J. C., Omidi S., Ainsworth T., Simon S., Ulevitch R. J., Tobias P. S. Interactions of lipopolysaccharide with neutrophils in blood via CD14. J Leukoc Biol. 1993 May;53(5):518–524. doi: 10.1002/jlb.53.5.518. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Hermanowski-Vosatka A., Rockwell P., Detmers P. A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991 May 1;173(5):1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagawa K., Kaku M., Ichinose Y., Nagao S., Tanaka A., Tomoda A. Biphasic effects of muramyl dipeptide or lipopolysaccharide on superoxide anion-generating activities of macrophages. Infect Immun. 1984 Jul;45(1):82–86. doi: 10.1128/iai.45.1.82-86.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J., Christou N. V. Neutrophil priming by lipopolysaccharide involves heterogeneity in calcium-mediated signal transduction. Studies using fluo-3 and flow cytometry. J Immunol. 1993 Mar 1;150(5):1988–1997. [PubMed] [Google Scholar]
- Yuo A., Kitagawa S., Kasahara T., Matsushima K., Saito M., Takaku F. Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood. 1991 Nov 15;78(10):2708–2714. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Fingerle G., Ströbel M., Schraut W., Stelter F., Schütt C., Passlick B., Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993 Sep;23(9):2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ströbel M., Kieper D., Fingerle G., Schlunck T., Petersmann I., Ellwart J., Blumenstein M., Haas J. G. Differential expression of cytokines in human blood monocyte subpopulations. Blood. 1992 Jan 15;79(2):503–511. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., Berg E. L., Michie S. A., Brown D. A., Karolak D., Ramezani L., Berger E. M., Arfors K. E., Butcher E. C. L-selectin mediates neutrophil rolling in inflamed venules through sialyl LewisX-dependent and -independent recognition pathways. Blood. 1993 Jul 1;82(1):182–191. [PubMed] [Google Scholar]


