Abstract
Hairpin oligonucleotides were synthesized with stems ending in a double-stranded structure, which can be ligated to double-strand breaks in DNA, and with loops that contain nucleotides modified by the attachment of biotin. These probes specifically and sensitively detect double-strand breaks in apoptotic cells. Localization of these probes is restricted to areas of chromatin characteristic of apoptosis, whereas much more diffuse labeling was obtained when all available 3' DNA ends were labeled by terminal transferase. In principle, hairpin oligonucleotide probes can be designed with any type of 3' or 5' overhang complementary to double-strand DNA termini being detected.
Full text
PDF

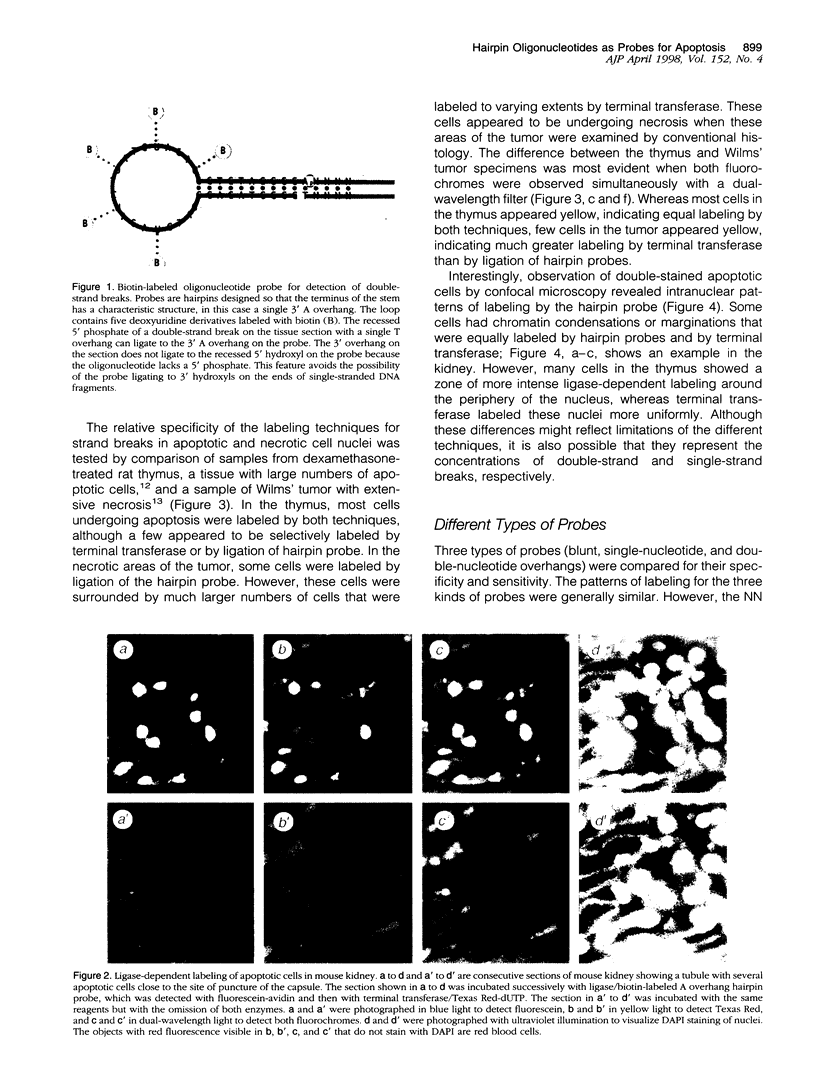



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Litwack G. Activation of internucleosomal DNA cleavage in human CEM lymphocytes by glucocorticoid and novobiocin. Evidence for a non-Ca2(+)-requiring mechanism(s). J Biol Chem. 1990 Oct 5;265(28):17323–17333. [PubMed] [Google Scholar]
- Beletsky I. P., Matyásová J., Nikonova L. V., Skalka M., Umansky S. R. On the role of Ca, Mg-dependent nuclease in the postirradiation degradation of chromatin in lymphoid tissues. Gen Physiol Biophys. 1989 Aug;8(4):381–398. [PubMed] [Google Scholar]
- Columbano A. Cell death: current difficulties in discriminating apoptosis from necrosis in the context of pathological processes in vivo. J Cell Biochem. 1995 Jun;58(2):181–190. doi: 10.1002/jcb.240580207. [DOI] [PubMed] [Google Scholar]
- Didenko V. V., Hornsby P. J. Presence of double-strand breaks with single-base 3' overhangs in cells undergoing apoptosis but not necrosis. J Cell Biol. 1996 Dec;135(5):1369–1376. doi: 10.1083/jcb.135.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Saikumar P., Weinberg J. M., Venkatachalam M. A. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement of serine but not cysteine proteases. Am J Pathol. 1997 Nov;151(5):1205–1213. [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C. Nuclear changes in apoptosis. Curr Opin Cell Biol. 1995 Jun;7(3):337–343. doi: 10.1016/0955-0674(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Edelstein C. L., Ling H., Schrier R. W. The nature of renal cell injury. Kidney Int. 1997 May;51(5):1341–1351. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- Enari M., Sakahira H., Yokoyama H., Okawa K., Iwamatsu A., Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998 Jan 1;391(6662):43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Gates G. F., Miller J. H., Stanley P. Necrosis of Wilms tumors. J Urol. 1980 Jun;123(6):916–920. doi: 10.1016/s0022-5347(17)56192-3. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D. Defining apoptosis. Am J Pathol. 1995 Jan;146(1):16–19. [PMC free article] [PubMed] [Google Scholar]
- Lieberthal W., Levine J. S. Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol. 1996 Sep;271(3 Pt 2):F477–F488. doi: 10.1152/ajprenal.1996.271.3.F477. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Mannherz H. G., Peitsch M. C., Zanotti S., Paddenberg R., Polzar B. A new function for an old enzyme: the role of DNase I in apoptosis. Curr Top Microbiol Immunol. 1995;198:161–174. doi: 10.1007/978-3-642-79414-8_10. [DOI] [PubMed] [Google Scholar]
- Nikonova L. V., Nelipovich P. A., Umansky S. R. The involvement of nuclear nucleases in rat thymocyte DNA degradation after gamma-irradiation. Biochim Biophys Acta. 1982 Dec 31;699(3):281–289. doi: 10.1016/0167-4781(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Müller C., Tschopp J. DNA fragmentation during apoptosis is caused by frequent single-strand cuts. Nucleic Acids Res. 1993 Sep 11;21(18):4206–4209. doi: 10.1093/nar/21.18.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzar B., Peitsch M. C., Loos R., Tschopp J., Mannherz H. G. Overexpression of deoxyribonuclease I (DNase I) transfected into COS-cells: its distribution during apoptotic cell death. Eur J Cell Biol. 1993 Dec;62(2):397–405. [PubMed] [Google Scholar]
- Potten C. S. What is an apoptotic index measuring? A commentary. Br J Cancer. 1996 Dec;74(11):1743–1748. doi: 10.1038/bjc.1996.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. Apoptosis and renal injury. Curr Opin Nephrol Hypertens. 1995 May;4(3):263–269. doi: 10.1097/00041552-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Melchior W., Jr, Felsenfeld G. DNAase I, DNAase II and staphylococcal nuclease cut at different, yet symmetrically located, sites in the nucleosome core. Cell. 1978 Jul;14(3):611–627. doi: 10.1016/0092-8674(78)90246-5. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]






