Abstract
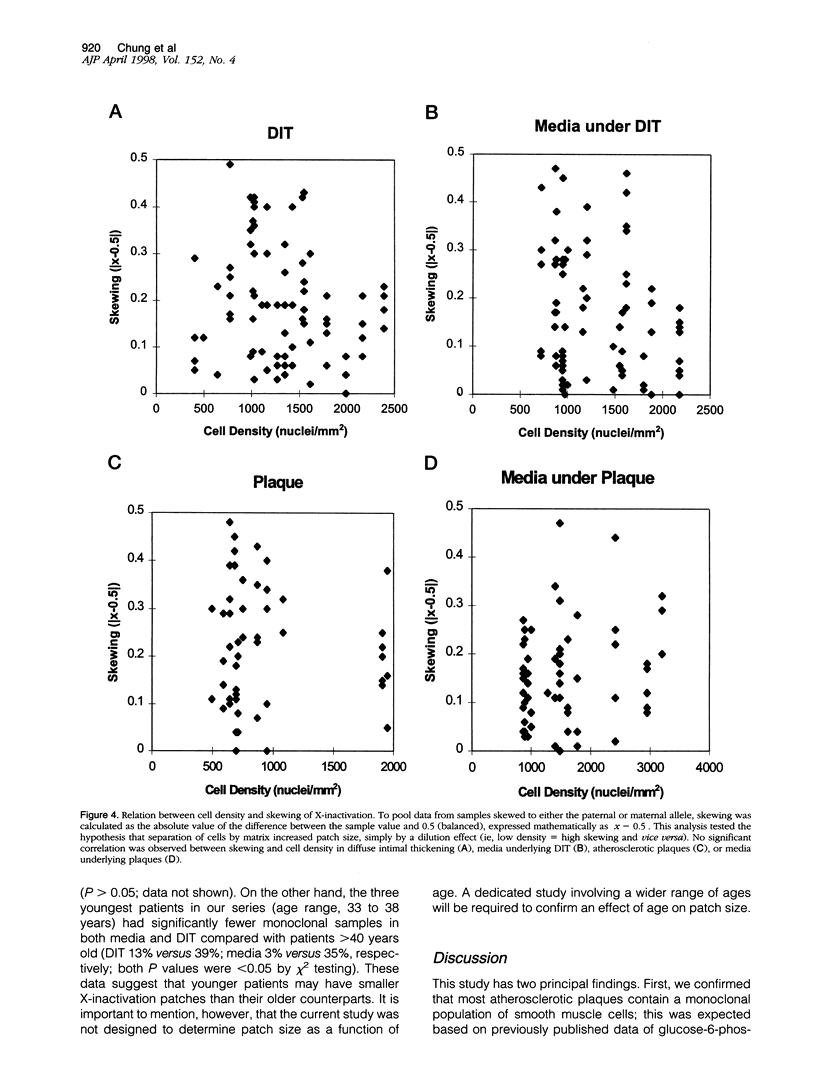
X chromosome inactivation studies indicate that smooth muscle cells in human atherosclerosis are monoclonal. Monoclonality could arise either by 1) proliferation of a single cell in the adult intima, eg, by selection or mutation, or 2) proliferation of many cells within a large, pre-existing clonal patch that formed during development. To determine whether clonal expansion occurs concomitantly with plaque growth or as part of normal development, X chromosome inactivation patterns were mapped in microdissected samples of aortic smooth muscle, using the human androgen receptor locus as a marker. As expected, 43% of plaque samples were skewed toward one X chromosome, indicating a monoclonal population. Surprisingly, 25% of normal medial samples and 31% of diffuse intimal thickening samples also were skewed toward one X chromosome, indicating a relatively large patch size. Furthermore, 30% of diffuse intimal thickening and 22% of medial samples showed contiguous regions of 4 mm skewed to the same allele, showing that patch length often exceeded 4 mm. Intima and overlying media typically were skewed to the same allele (73% concordance), suggesting common cells of origin. Because patch size is large in normal arteries, X-inactivation analysis cannot discriminate between a monoclonal and a polyclonal origin of plaque smooth muscle cells. We propose that human arteries grow by expanding coherent smooth muscle clones, with little mixing of adjacent clones. Determining whether plaques arise by clonal expansion will require other approaches, such as analysis of somatic mutations; the finding of large X-inactivation patches raises the possibility that plaques arise from a pre-existing (developmental) clone.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalone R., Granata P., Minelli E., Portentoso P., Giudici A., Righi R., Castelli P., Socrate A., Frigerio B. Cytogenetic analysis reveals clonal proliferation of smooth muscle cells in atherosclerotic plaques. Hum Genet. 1991 Jun;87(2):139–143. doi: 10.1007/BF00204169. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. The origin and development of human tumors studied with cell markers. N Engl J Med. 1974 Jul 4;291(1):26–35. doi: 10.1056/NEJM197407042910109. [DOI] [PubMed] [Google Scholar]
- Frid M. G., Moiseeva E. P., Stenmark K. R. Multiple phenotypically distinct smooth muscle cell populations exist in the adult and developing bovine pulmonary arterial media in vivo. Circ Res. 1994 Oct;75(4):669–681. doi: 10.1161/01.res.75.4.669. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hatzistamou J., Kiaris H., Ergazaki M., Spandidos D. A. Loss of heterozygosity and microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996 Aug 5;225(1):186–190. doi: 10.1006/bbrc.1996.1151. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M. The study of mammalian organogenesis by mosaic pattern analysis. Cell Differ. 1987 Jul;21(2):79–91. doi: 10.1016/0045-6039(87)90415-5. [DOI] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Covin C. W., White S., Giachelli C. M., Schwartz S. M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994 May;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Linder D., Gartler S. M. Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science. 1965 Oct 1;150(3692):67–69. doi: 10.1126/science.150.3692.67. [DOI] [PubMed] [Google Scholar]
- Lindner V., Giachelli C. M., Schwartz S. M., Reidy M. A. A subpopulation of smooth muscle cells in injured rat arteries expresses platelet-derived growth factor-B chain mRNA. Circ Res. 1995 Jun;76(6):951–957. doi: 10.1161/01.res.76.6.951. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Microsatellite instability: marker of a mutator phenotype in cancer. Cancer Res. 1994 Oct 1;54(19):5059–5063. [PubMed] [Google Scholar]
- Mashal R. D., Lester S. C., Sklar J. Clonal analysis by study of X chromosome inactivation in formalin-fixed paraffin-embedded tissue. Cancer Res. 1993 Oct 1;53(19):4676–4679. [PubMed] [Google Scholar]
- McCaffrey T. A., Du B., Consigli S., Szabo P., Bray P. J., Hartner L., Weksler B. B., Sanborn T. A., Bergman G., Bush H. L., Jr Genomic instability in the type II TGF-beta1 receptor gene in atherosclerotic and restenotic vascular cells. J Clin Invest. 1997 Nov 1;100(9):2182–2188. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T., Fischman D. A. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry C. E., Bartosek T., Giachelli C. M., Alpers C. E., Schwartz S. M. Platelet-derived growth factor-A mRNA expression in fetal, normal adult, and atherosclerotic human aortas. Analysis by competitive polymerase chain reaction. Circulation. 1996 Mar 15;93(6):1095–1106. doi: 10.1161/01.cir.93.6.1095. [DOI] [PubMed] [Google Scholar]
- Murry C. E., Gipaya C. T., Bartosek T., Benditt E. P., Schwartz S. M. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997 Sep;151(3):697–705. [PMC free article] [PubMed] [Google Scholar]
- Nesbitt M. N. Chimeras vs X inactivation mosaics: significance of differences in pigment distribution. Dev Biol. 1974 May;38(1):202–207. doi: 10.1016/0012-1606(74)90272-3. [DOI] [PubMed] [Google Scholar]
- Ng Y. K., Ohaki Y., Deamant F., Iannaccone P. M. Comparison of epidermal patch size in X-chromosome-linked mosaic and dizygotic chimeric mice. Cell Differ Dev. 1990 Apr;30(1):27–34. doi: 10.1016/0922-3371(90)90071-4. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Heptinstall R. H. Clonal mapping of the human aorta. Relationship of monoclonal characteristics, lesion thickness, and age in normal intima and atherosclerotic lesions. Am J Pathol. 1987 Jan;126(1):33–39. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Heptinstall R. H. The clonal characteristics of human aortic intima. Comparison with fatty streaks and normal media. Am J Pathol. 1983 Oct;113(1):33–40. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solex K., Heptinstall R. H. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978 Jul;43(1):10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solez K., Heptinstall R. H. Clonal characteristics of cutaneous scars and implications for atherogenesis. Am J Pathol. 1981 Jan;102(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Pearson T. A., Dillman J., Solez K., Heptinstall R. H. Monoclonal characteristics of organising arterial thrombi: Significance in the origin and growth of human atherosclerotic plaques. Lancet. 1979 Jan 6;1(8106):7–11. doi: 10.1016/s0140-6736(79)90453-7. [DOI] [PubMed] [Google Scholar]
- Penn A., Snyder C. Arteriosclerotic plaque development is 'promoted' by polynuclear aromatic hydrocarbons. Carcinogenesis. 1988 Dec;9(12):2185–2189. doi: 10.1093/carcin/9.12.2185. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., deBlois D., O'Brien E. R. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995 Sep;77(3):445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Sims F. H., Gavin J. B., Vanderwee M. A. The intima of human coronary arteries. Am Heart J. 1989 Jul;118(1):32–38. doi: 10.1016/0002-8703(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Ergazaki M., Arvanitis D., Kiaris H. Microsatellite instability in human atherosclerotic plaques. Biochem Biophys Res Commun. 1996 Mar 7;220(1):137–140. doi: 10.1006/bbrc.1996.0370. [DOI] [PubMed] [Google Scholar]
- Thomas W. A., Reiner J. M., Janakidevi K., Florentin R. A., Lee K. T. Population dynamics of arterial cells during atherogenesis. X. Study of monotypism in atherosclerotic lesions of black women heterozygous for glucose-6-phosphate dehydrogenase (G-6-PD). Exp Mol Pathol. 1979 Dec;31(3):367–386. doi: 10.1016/0014-4800(79)90038-8. [DOI] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman T. G. Enzyme patterns in tetraparental mouse liver. Nature. 1970 Jan 31;225(5231):462–463. doi: 10.1038/225462a0. [DOI] [PubMed] [Google Scholar]



