Abstract
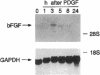
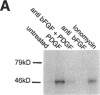
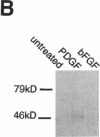
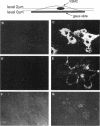
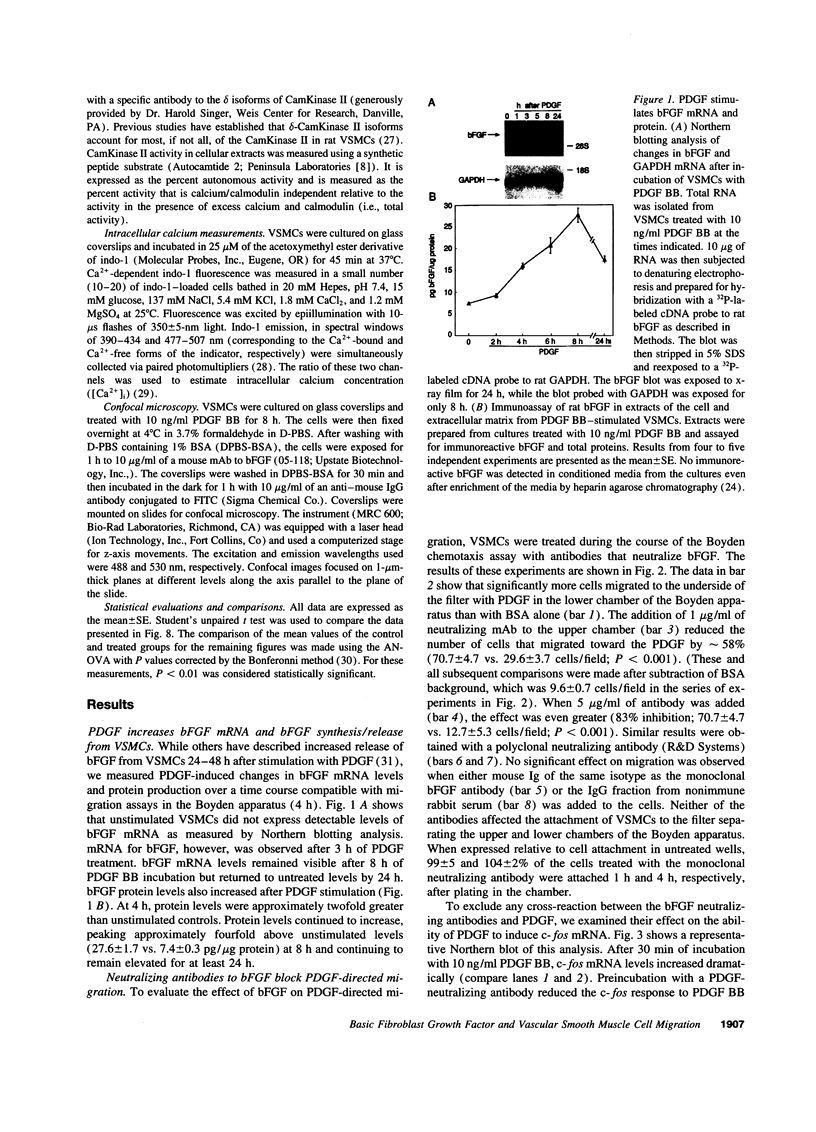
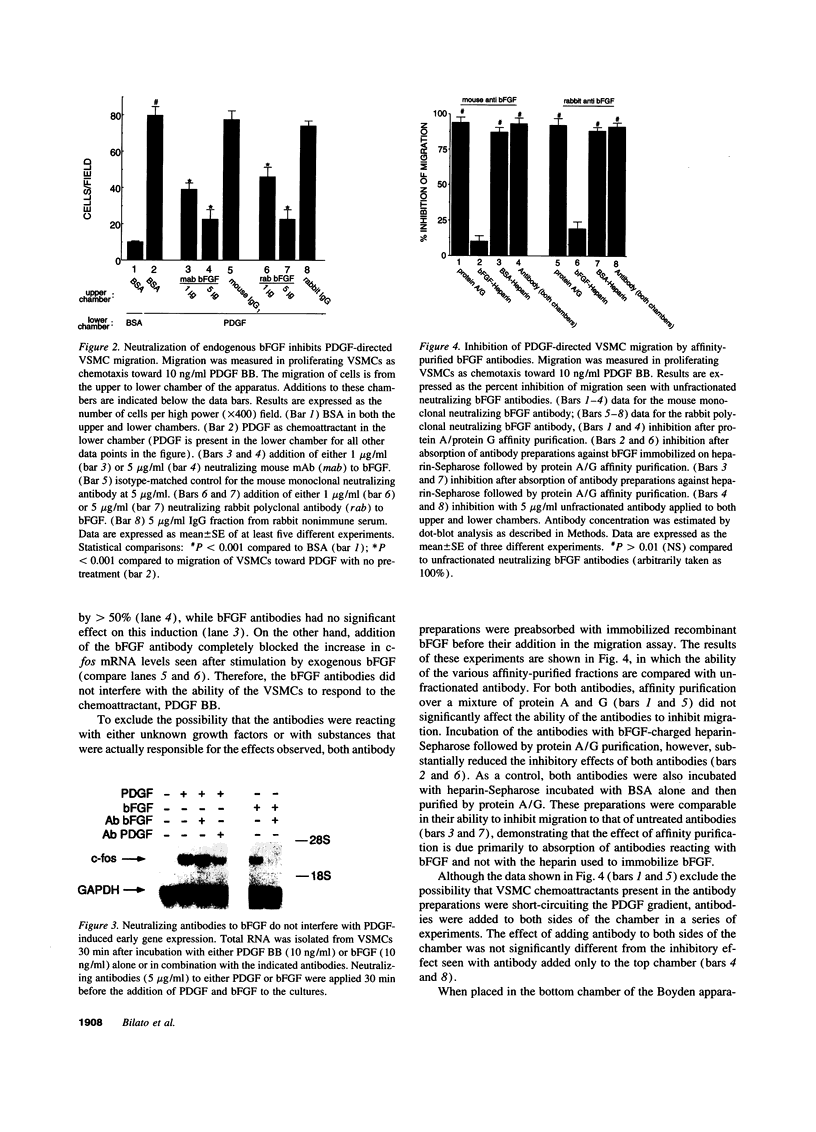
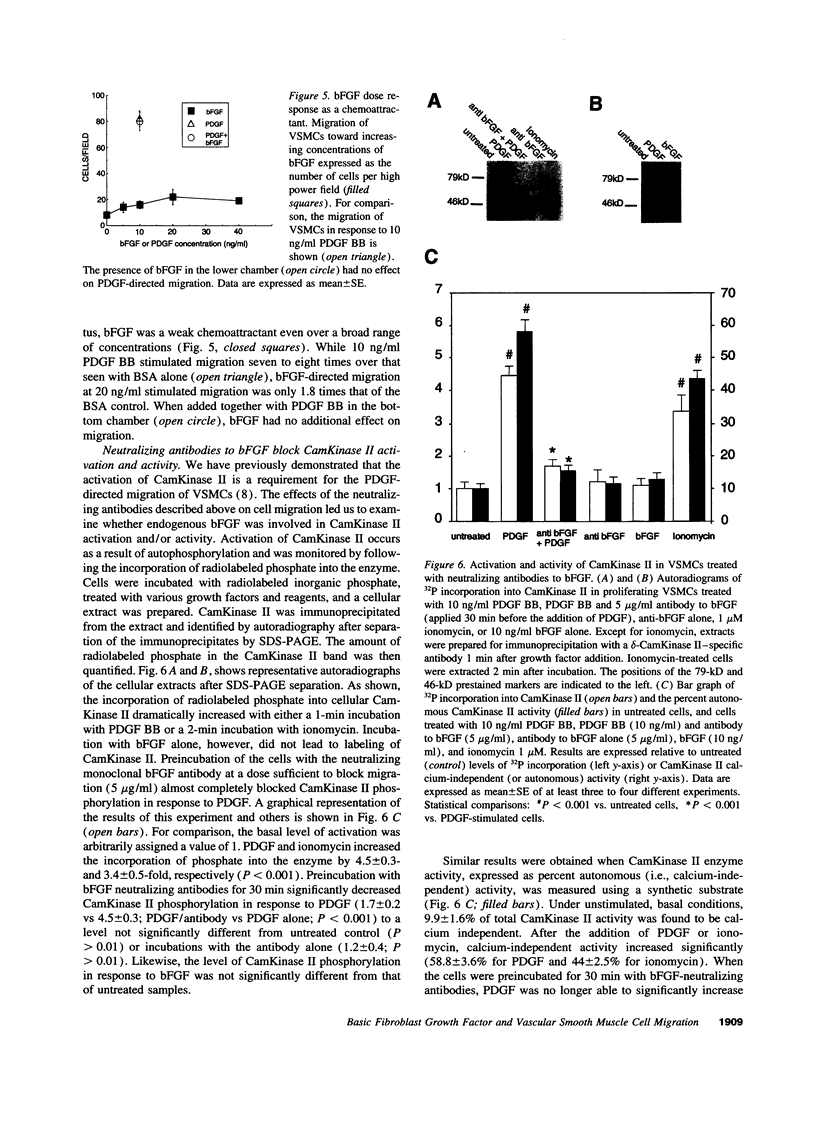
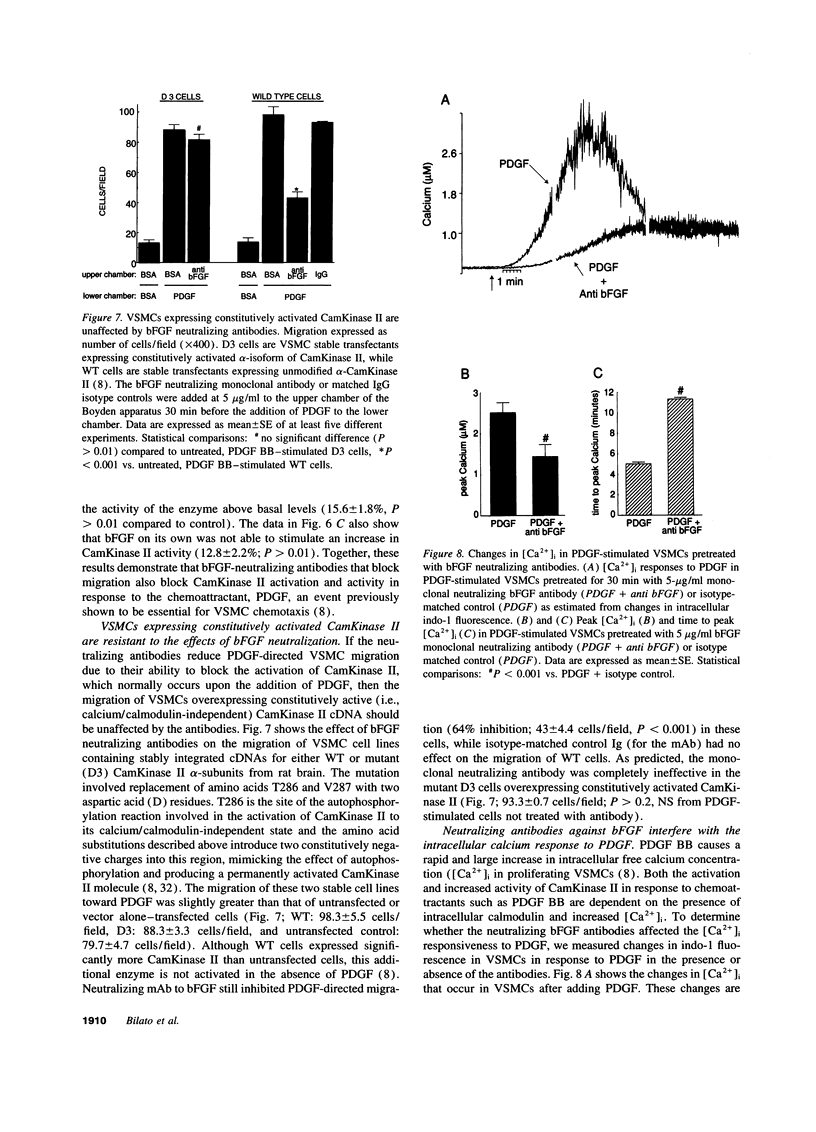
Intracellular signaling pathways activated by both PDGF and basic fibroblast growth factor (bFGF) have been implicated in the migration of vascular smooth muscle cells (VSMC), a key step in the pathogenesis of many vascular diseases. We demonstrate here that, while bFGF is a weak chemoattractant for VSMCs, it is required for the PDGF-directed migration of VSMCs and the activation of calcium/calmodulin-dependent protein kinase II (CamKinase II), an intracellular event that we have previously shown to be important in the regulation of VSMC migration. Neutralizing antibodies to bFGF caused a dramatic reduction in the size of the intracellular calcium transient normally seen after PDGF stimulation and inhibited both PDGF-directed VSMC migration and CamKinase II activation. Partially restoring the calcium transient with ionomycin restored migration and CamKinase II activation as did the forced expression of a mutant CamKinase II that had been "locked" in the active state by site-directed mutagenesis. These results suggest that bFGF links PDGF receptor stimulation to changes in intracellular calcium and CamKinase II activation, reinforcing the central role played by CamKinase II in regulating VSMC migration.
Full text
PDF

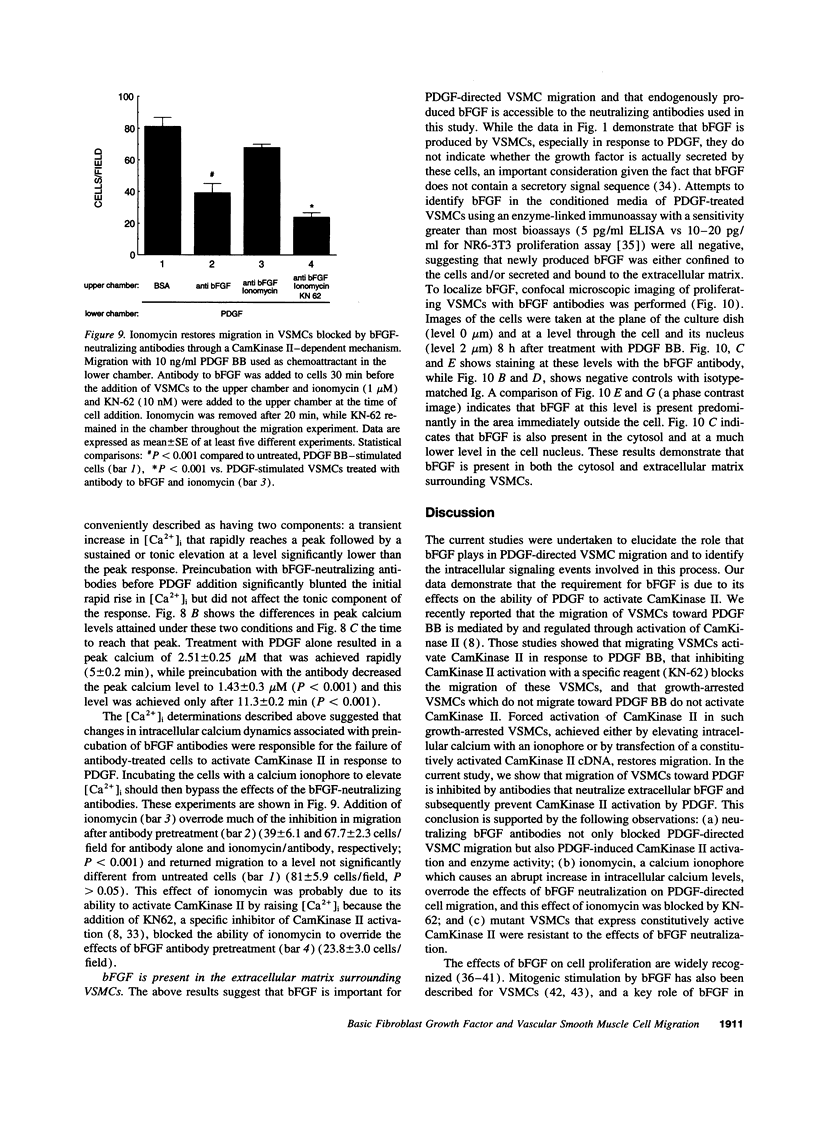
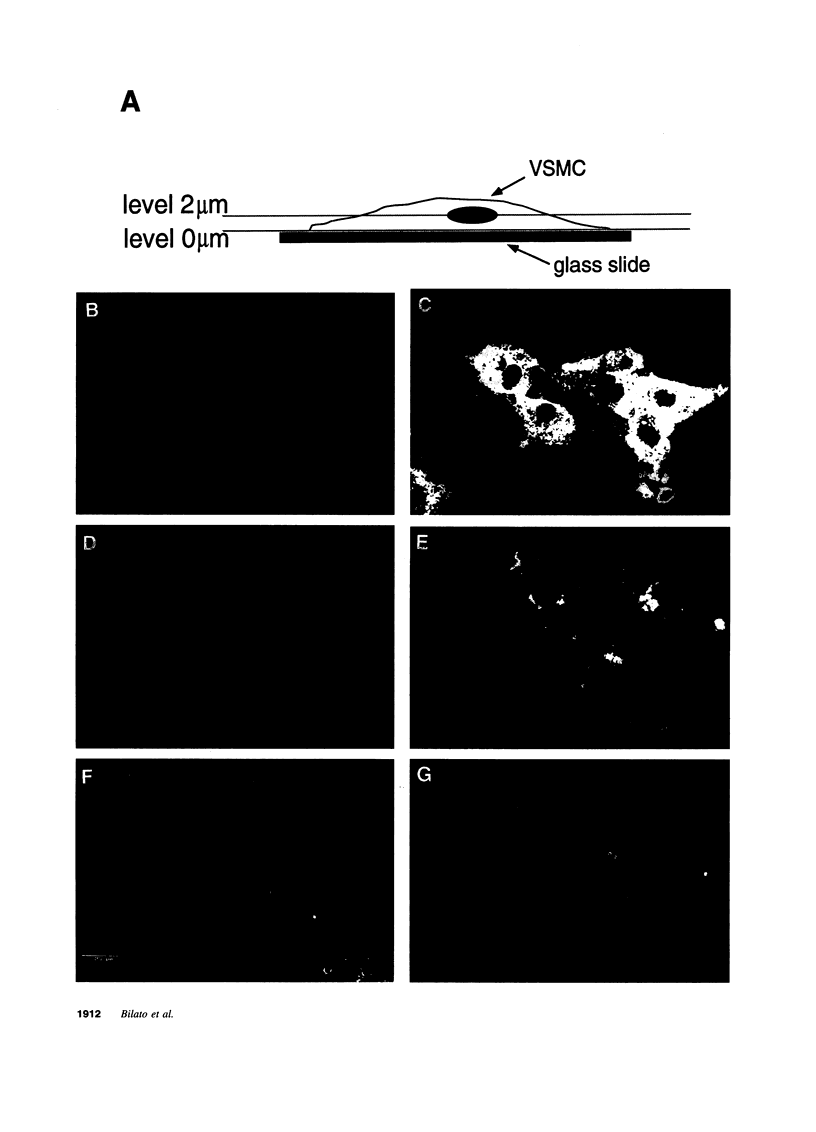



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Alberts G. F., Hsu D. K., Peifley K. A., Winkles J. A. Differential regulation of acidic and basic fibroblast growth factor gene expression in fibroblast growth factor-treated rat aortic smooth muscle cells. Circ Res. 1994 Aug;75(2):261–267. doi: 10.1161/01.res.75.2.261. [DOI] [PubMed] [Google Scholar]
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Ali S., Davis M. G., Becker M. W., Dorn G. W., 2nd Thromboxane A2 stimulates vascular smooth muscle hypertrophy by up-regulating the synthesis and release of endogenous basic fibroblast growth factor. J Biol Chem. 1993 Aug 15;268(23):17397–17403. [PubMed] [Google Scholar]
- Baird A., Klagsbrun M. The fibroblast growth factor family. Cancer Cells. 1991 Jun;3(6):239–243. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Biro S., Yu Z. X., Fu Y. M., Smale G., Sasse J., Sanchez J., Ferrans V. J., Casscells W. Expression and subcellular distribution of basic fibroblast growth factor are regulated during migration of endothelial cells. Circ Res. 1994 Mar;74(3):485–494. doi: 10.1161/01.res.74.3.485. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Connolly D. T., Stoddard B. L., Harakas N. K., Feder J. Human fibroblast-derived growth factor is a mitogen and chemoattractant for endothelial cells. Biochem Biophys Res Commun. 1987 Apr 29;144(2):705–712. doi: 10.1016/s0006-291x(87)80022-0. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Smith S. R. Growth factor effects on cells of the vascular wall: a survey. Growth Factors. 1993;8(1):61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Pozzan T., Wollheim C. B., Vicentini L. M., Meldolesi J. Tumor promoter phorbol myristate acetate inhibits Ca2+ influx through voltage-gated Ca2+ channels in two secretory cell lines, PC12 and RINm5F. J Biol Chem. 1986 Jan 5;261(1):32–35. [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992 May 1;69(3):413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek C. M., Luo Z., Mayberg M. R. Basic fibroblast growth factor and transforming growth factor beta-1: synergistic mediators of angiogenesis in vitro. J Cell Physiol. 1993 Oct;157(1):133–144. doi: 10.1002/jcp.1041570118. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Hirabayashi K., Giguère L., Tauber J. P. Factors controlling the proliferative rate, final cell density, and life span of bovine vascular smooth muscle cells in culture. J Cell Biol. 1981 Jun;89(3):568–578. doi: 10.1083/jcb.89.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Mescher A. L., Birdwell C. R. Stimulation of corneal endothelial cell proliferations in vitro by fibroblast and epidermal growth factors. Exp Eye Res. 1977 Jul;25(1):75–89. doi: 10.1016/0014-4835(77)90248-2. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Funasaka Y., Lee P., Rubin J., Ron D., Birnbaum D. Fibroblast growth factors in normal and malignant melanocytes. Ann N Y Acad Sci. 1991;638:232–243. doi: 10.1111/j.1749-6632.1991.tb49034.x. [DOI] [PubMed] [Google Scholar]
- Hughes S. E., Hall P. A. Overview of the fibroblast growth factor and receptor families: complexity, functional diversity, and implications for future cardiovascular research. Cardiovasc Res. 1993 Jul;27(7):1199–1203. doi: 10.1093/cvr/27.7.1199. [DOI] [PubMed] [Google Scholar]
- Jackson C. L., Reidy M. A. Basic fibroblast growth factor: its role in the control of smooth muscle cell migration. Am J Pathol. 1993 Oct;143(4):1024–1031. [PMC free article] [PubMed] [Google Scholar]
- Jawien A., Bowen-Pope D. F., Lindner V., Schwartz S. M., Clowes A. W. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992 Feb;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Lindner V., Reidy M. A. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3739–3743. doi: 10.1073/pnas.88.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol M., Jefferson A. B., Schulman H. Ca2+/calmodulin kinase is activated by the phosphatidylinositol signaling pathway and becomes Ca2(+)-independent in PC12 cells. J Biol Chem. 1990 Oct 25;265(30):18055–18058. [PubMed] [Google Scholar]
- MacNicol M., Schulman H. Cross-talk between protein kinase C and multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1992 Jun 15;267(17):12197–12201. [PubMed] [Google Scholar]
- Matsuzaki K., Yoshitake Y., Matuo Y., Sasaki H., Nishikawa K. Monoclonal antibodies against heparin-binding growth factor II/basic fibroblast growth factor that block its biological activity: invalidity of the antibodies for tumor angiogenesis. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9911–9915. doi: 10.1073/pnas.86.24.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignatti P., Morimoto T., Rifkin D. B. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol. 1992 Apr;151(1):81–93. doi: 10.1002/jcp.1041510113. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Tsuboi R., Robbins E., Rifkin D. B. In vitro angiogenesis on the human amniotic membrane: requirement for basic fibroblast growth factor-induced proteinases. J Cell Biol. 1989 Feb;108(2):671–682. doi: 10.1083/jcb.108.2.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Vassalli J. D., Baird A., Guillemin R., Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nånberg E., Morris C., Higgins T., Vara F., Rozengurt E. Fibroblast growth factor stimulates protein kinase C in quiescent 3T3 cells without Ca2+ mobilization or inositol phosphate accumulation. J Cell Physiol. 1990 May;143(2):232–242. doi: 10.1002/jcp.1041430206. [DOI] [PubMed] [Google Scholar]
- Pauly R. R., Bilato C., Sollott S. J., Monticone R., Kelly P. T., Lakatta E. G., Crow M. T. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation. 1995 Feb 15;91(4):1107–1115. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- Pauly R. R., Passaniti A., Bilato C., Monticone R., Cheng L., Papadopoulos N., Gluzband Y. A., Smith L., Weinstein C., Lakatta E. G. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994 Jul;75(1):41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- Powell P. P., Klagsbrun M. Three forms of rat basic fibroblast growth factor are made from a single mRNA and localize to the nucleus. J Cell Physiol. 1991 Aug;148(2):202–210. doi: 10.1002/jcp.1041480204. [DOI] [PubMed] [Google Scholar]
- Presta M., Tiberio L., Rusnati M., Dell'Era P., Ragnotti G. Basic fibroblast growth factor requires a long-lasting activation of protein kinase C to induce cell proliferation in transformed fetal bovine aortic endothelial cells. Cell Regul. 1991 Sep;2(9):719–726. doi: 10.1091/mbc.2.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzino A., Kazakoff P., Ruff E., Kuszynski C., Nebelsick J. Regulatory effects of cell density on the binding of transforming growth factor beta, epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor. Cancer Res. 1988 Aug 1;48(15):4266–4271. [PubMed] [Google Scholar]
- Rogelj S., Klagsbrun M., Atzmon R., Kurokawa M., Haimovitz A., Fuks Z., Vlodavsky I. Basic fibroblast growth factor is an extracellular matrix component required for supporting the proliferation of vascular endothelial cells and the differentiation of PC12 cells. J Cell Biol. 1989 Aug;109(2):823–831. doi: 10.1083/jcb.109.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Hamanaka R., Ono J., Kuwano M., Rifkin D. B., Takaki R. The stimulatory effect of PDGF on vascular smooth muscle cell migration is mediated by the induction of endogenous basic FGF. Biochem Biophys Res Commun. 1991 Feb 14;174(3):1260–1266. doi: 10.1016/0006-291x(91)91557-s. [DOI] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schworer C. M., Rothblum L. I., Thekkumkara T. J., Singer H. A. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. J Biol Chem. 1993 Jul 5;268(19):14443–14449. [PubMed] [Google Scholar]
- Shimasaki S., Emoto N., Koba A., Mercado M., Shibata F., Cooksey K., Baird A., Ling N. Complementary DNA cloning and sequencing of rat ovarian basic fibroblast growth factor and tissue distribution study of its mRNA. Biochem Biophys Res Commun. 1988 Nov 30;157(1):256–263. doi: 10.1016/s0006-291x(88)80041-x. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Brock T. A. Analysis of angiotensin-stimulated sodium transport in cultured smooth muscle cells from rat aorta. J Cell Physiol. 1983 Mar;114(3):284–290. doi: 10.1002/jcp.1041140306. [DOI] [PubMed] [Google Scholar]
- Spurgeon H. A., Stern M. D., Baartz G., Raffaeli S., Hansford R. G., Talo A., Lakatta E. G., Capogrossi M. C. Simultaneous measurement of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990 Feb;258(2 Pt 2):H574–H586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990 Mar 15;265(8):4315–4320. [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Westgate S. A., Kelly P. T. Mutagenesis of Thr-286 in monomeric Ca2+/calmodulin-dependent protein kinase II eliminates Ca2+/calmodulin-independent activity. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1273–1277. doi: 10.1073/pnas.87.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weich H. A., Iberg N., Klagsbrun M., Folkman J. Expression of acidic and basic fibroblast growth factors in human and bovine vascular smooth muscle cells. Growth Factors. 1990;2(4):313–320. doi: 10.3109/08977199009167026. [DOI] [PubMed] [Google Scholar]
- Zheng J. S., Boluyt M. O., O'Neill L., Crow M. T., Lakatta E. G. Extracellular ATP induces immediate-early gene expression but not cellular hypertrophy in neonatal cardiac myocytes. Circ Res. 1994 Jun;74(6):1034–1041. doi: 10.1161/01.res.74.6.1034. [DOI] [PubMed] [Google Scholar]
- Ziegelstein R. C., Cheng L., Blank P. S., Spurgeon H. A., Lakatta E. G., Hansford R. G., Capogrossi M. C. Modulation of calcium homeostasis in cultured rat aortic endothelial cells by intracellular acidification. Am J Physiol. 1993 Oct;265(4 Pt 2):H1424–H1433. doi: 10.1152/ajpheart.1993.265.4.H1424. [DOI] [PubMed] [Google Scholar]