Abstract
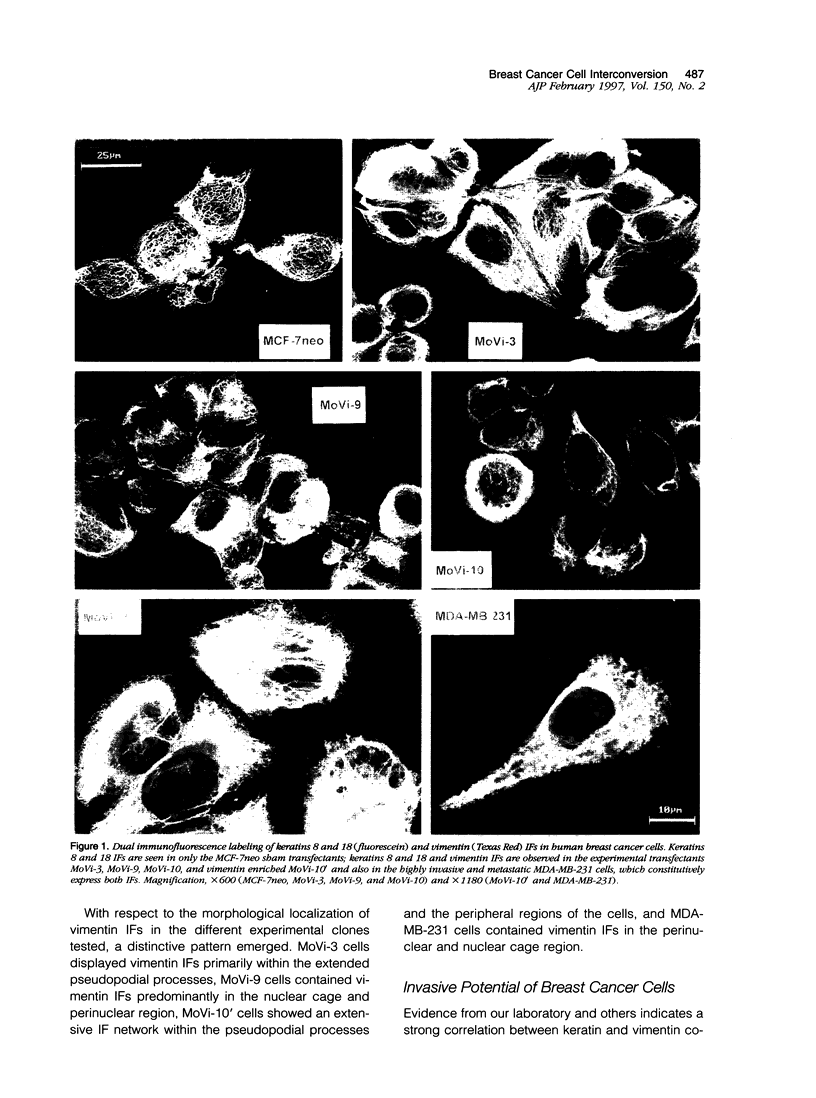
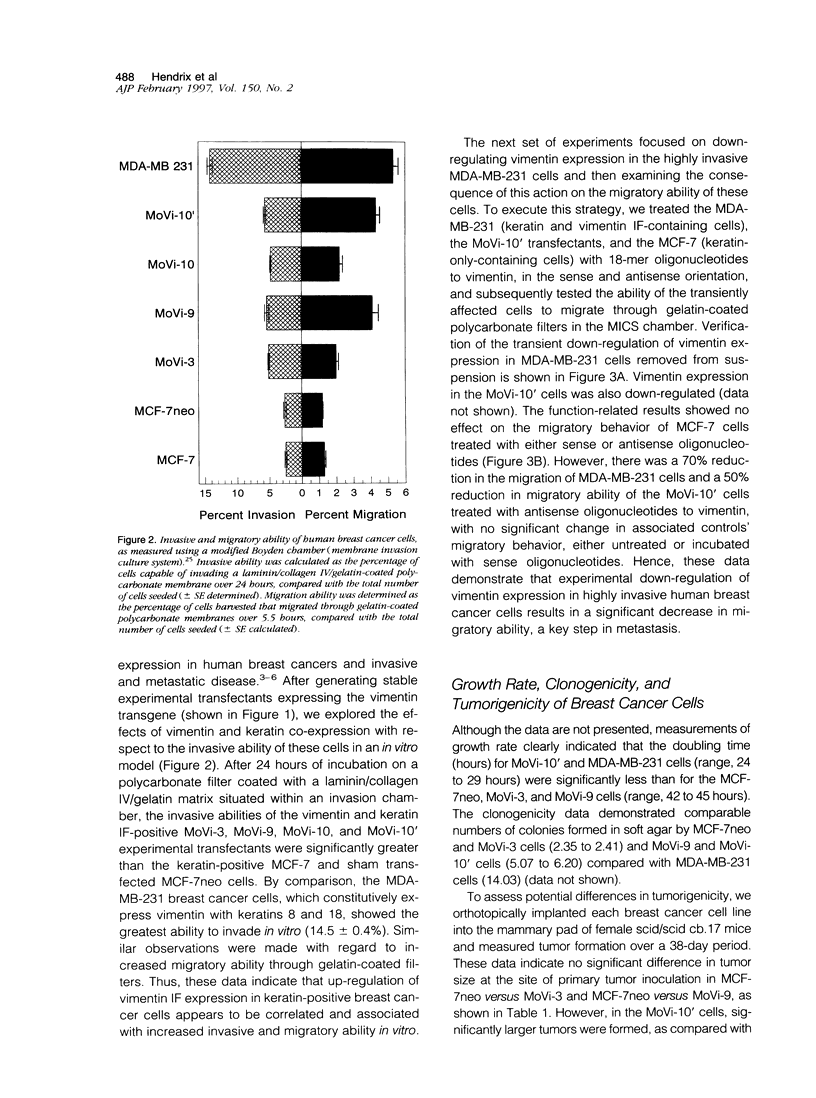
The expression of intermediate filament proteins is remarkably tissue specific, which suggests that the intermediate filament type(s) present in cells is somehow related to their biological function. However, in some cancers, particularly malignant breast carcinoma, there is a strong indication that vimentin is co-expressed with keratins, thus presenting as a dedifferentiated or interconverted (between epithelial and mesenchymal) phenotype. In the present study, we recapitulated the interconverted phenotype by developing stable transfectants of MCF-7 human breast cancer cells, termed MoVi clones, to express both vimentin and keratins. Overexpression of vimentin in these cells led to augmentation of motility and invasiveness in vitra. These activities could be transiently down-regulated by vimentin antisense oligonucleotides in MoVi clones and MDA-MB-231 cells (which constitutively co-express keratins and vimentin). Furthermore, in the MoVi experimental transfectants expressing the highest percentage of vimentin-positive cells, their proliferative capacity, clonogenic potential, and tumorigenicity increased. However, the metastatic ability of the MoVi transfectants remained unchanged compared with MCF-7neo controls. The MDA-MB-231 cells metastasized to axillary lymph nodes in a SCID mouse model. Finally, we explored the possibility that potential changes could occur with respect to cell surface integrins. These studies revealed a decrease in the alpha 2- and alpha 3-containing promiscuous integrins, in addition to beta 1 containing integrins, concomitant with an increase in the alpha 6-containing laminin receptor integrin. Further functional analysis of the alpha 6 observation showed an increase in the baptotactic migration of MoVi transfectants toward a laminin substrate. From these data, it is postulated that the ability to co-express vimentin and keratins confers a selective advantage to breast cancer cells in their interpretation of signaling cues from the extracellular matrix; however the addition of vimentin intermediate filaments alone is not sufficient to confer the metastatic phenotype.
Full text
PDF



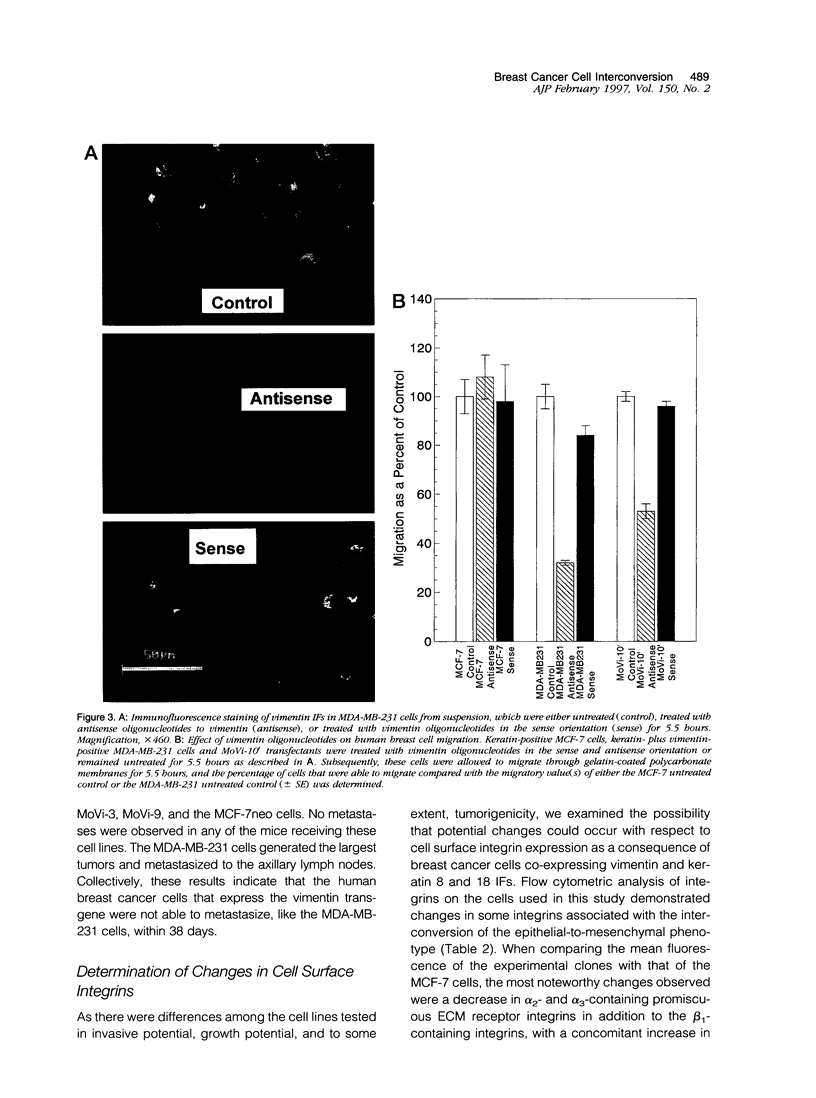
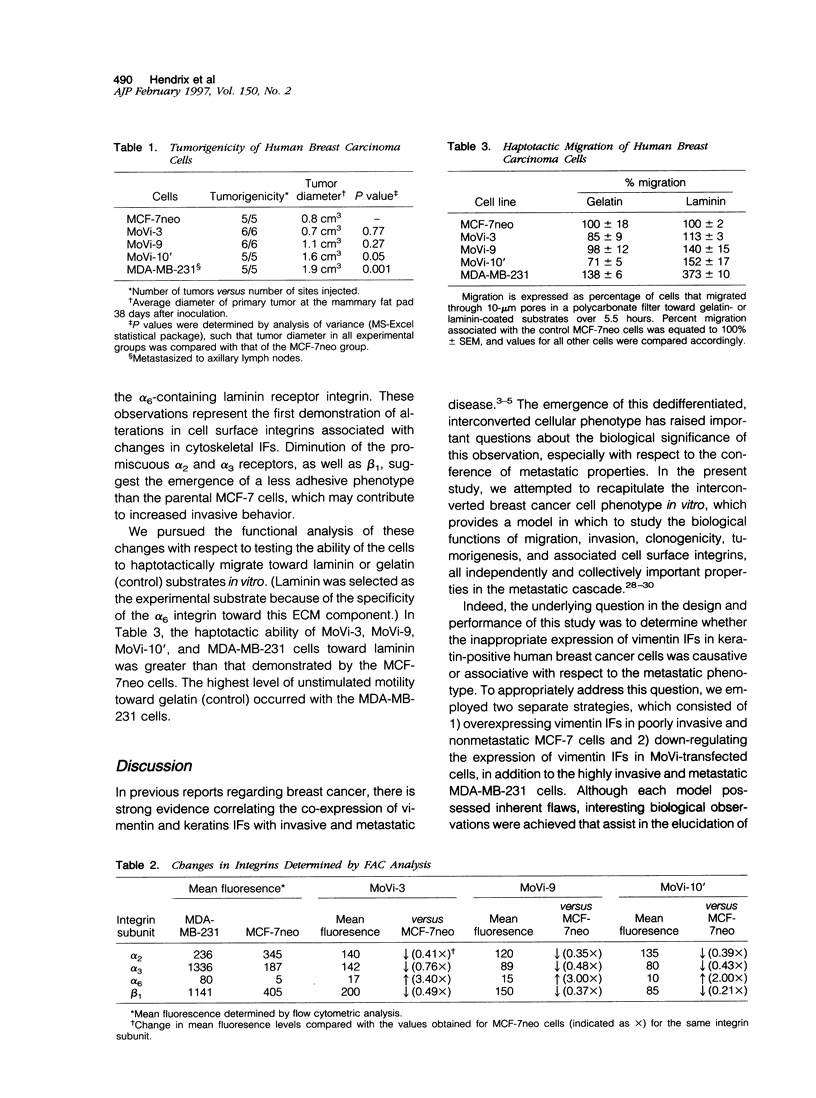





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Andreoli J. M., Trevor K. T. Structural and biological consequences of increased vimentin expression in simple epithelial cell types. Cell Motil Cytoskeleton. 1995;32(1):10–25. doi: 10.1002/cm.970320103. [DOI] [PubMed] [Google Scholar]
- Bauman P. A., Dalton W. S., Anderson J. M., Cress A. E. Expression of cytokeratin confers multiple drug resistance. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5311–5314. doi: 10.1073/pnas.91.12.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T. Cancer statistics, 1993. CA Cancer J Clin. 1993 Jan-Feb;43(1):7–26. doi: 10.3322/canjclin.43.1.7. [DOI] [PubMed] [Google Scholar]
- Chu Y. W., Runyan R. B., Oshima R. G., Hendrix M. J. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4261–4265. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. W., Seftor E. A., Romer L. H., Hendrix M. J. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol. 1996 Jan;148(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Domagala W., Lasota J., Bartkowiak J., Weber K., Osborn M. Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki-67 growth fraction. Am J Pathol. 1990 Jan;136(1):219–227. [PMC free article] [PubMed] [Google Scholar]
- Domagala W., Woźniak L., Lasota J., Weber K., Osborn M. Vimentin is preferentially expressed in high-grade ductal and medullary, but not in lobular breast carcinomas. Am J Pathol. 1990 Nov;137(5):1059–1064. [PMC free article] [PubMed] [Google Scholar]
- Duprey P., Morello D., Vasseur M., Babinet C., Condamine H., Brûlet P., Jacob F. Expression of the cytokeratin endo A gene during early mouse embryogenesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8535–8539. doi: 10.1073/pnas.82.24.8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Intermediate filaments and disease: mutations that cripple cell strength. J Cell Biol. 1994 May;125(3):511–516. doi: 10.1083/jcb.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Ginsberg M. H., Du X., Plow E. F. Inside-out integrin signalling. Curr Opin Cell Biol. 1992 Oct;4(5):766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. J., Goldman R. D. Evidence for an interaction between the cell surface and intermediate filaments in cultured fibroblasts. Cell Motil Cytoskeleton. 1986;6(4):389–405. doi: 10.1002/cm.970060405. [DOI] [PubMed] [Google Scholar]
- Griffith C. M., Hay E. D. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992 Dec;116(4):1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Gunning P., Leavitt J., Muscat G., Ng S. Y., Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4831–4835. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Chu Y. W., Seftor R. E., Nagle R. B., McDaniel K. M., Leong S. P., Yohem K. H., Leibovitz A. M., Meyskens F. L., Jr Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992 Feb 5;84(3):165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Fidler I. J. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987 Dec;38(1-2):137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol. 1991 Oct;3(5):841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkowsky M. W. Intermediate filaments: new proteins, some answers, more questions. Curr Opin Cell Biol. 1995 Feb;7(1):46–54. doi: 10.1016/0955-0674(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Kohn E. C., Liotta L. A. Molecular insights into cancer invasion: strategies for prevention and intervention. Cancer Res. 1995 May 1;55(9):1856–1862. [PubMed] [Google Scholar]
- Kubota S., Tashiro K., Yamada Y. Signaling site of laminin with mitogenic activity. J Biol Chem. 1992 Mar 5;267(7):4285–4288. [PubMed] [Google Scholar]
- Lane E. B., Hogan B. L., Kurkinen M., Garrels J. I. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983 Jun 23;303(5919):701–704. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- Lichtner R. B., Julian J. A., North S. M., Glasser S. R., Nicolson G. L. Coexpression of cytokeratins characteristic for myoepithelial and luminal cell lineages in rat 13762NF mammary adenocarcinoma tumors and their spontaneous metastases. Cancer Res. 1991 Nov 1;51(21):5943–5950. [PubMed] [Google Scholar]
- Liotta L. A., Steeg P. S., Stetler-Stevenson W. G. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991 Jan 25;64(2):327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- Lynch R. G. Differentiation and cancer: the conditional autonomy of phenotype. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):647–648. doi: 10.1073/pnas.92.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Palm S. L., Furcht L. T. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983 Sep;97(3):772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean W. H., Lane E. B. Intermediate filaments in disease. Curr Opin Cell Biol. 1995 Feb;7(1):118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Coso O. A., Gutkind J. S., Burbelo P. D., Akiyama S. K., Yamada K. M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995 Nov;131(3):791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Oshima R. G., Howe W. E., Klier F. G., Adamson E. D., Shevinsky L. H. Intermediate filament protein synthesis in preimplantation murine embryos. Dev Biol. 1983 Oct;99(2):447–455. doi: 10.1016/0012-1606(83)90294-4. [DOI] [PubMed] [Google Scholar]
- Oshima R. G. Intermediate filament molecular biology. Curr Opin Cell Biol. 1992 Feb;4(1):110–116. doi: 10.1016/0955-0674(92)90067-m. [DOI] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta V., Jones J. C. The internal affairs of an integrin. Trends Cell Biol. 1991 Jul;1(1):2–4. doi: 10.1016/0962-8924(91)90046-c. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Vimentin--a new prognostic parameter in breast carcinoma? J Pathol. 1989 Jun;158(2):107–114. doi: 10.1002/path.1711580205. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Nigam S. K., Goldberg I. D. Scatter factor and the c-met receptor: a paradigm for mesenchymal/epithelial interaction. J Cell Biol. 1994 Dec;127(6 Pt 2):1783–1787. doi: 10.1083/jcb.127.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarria A. J., Nordeen S. K., Evans R. M. Regulated expression of vimentin cDNA in cells in the presence and absence of a preexisting vimentin filament network. J Cell Biol. 1990 Aug;111(2):553–565. doi: 10.1083/jcb.111.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Gehlsen K. R., Stetler-Stevenson W. G., Brown P. D., Ruoslahti E., Hendrix M. J. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea T. B., Beermann M. L., Fischer I. Transient requirement for vimentin in neuritogenesis: intracellular delivery of anti-vimentin antibodies and antisense oligonucleotides inhibit neurite initiation but not elongation of existing neurites in neuroblastoma. J Neurosci Res. 1993 Sep 1;36(1):66–76. doi: 10.1002/jnr.490360108. [DOI] [PubMed] [Google Scholar]
- Skalli O., Goldman R. D. Recent insights into the assembly, dynamics, and function of intermediate filament networks. Cell Motil Cytoskeleton. 1991;19(2):67–79. doi: 10.1002/cm.970190202. [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Heckford S. E., Skerker J. M., Worland P., Torri J. A., Thompson E. W., Byers S. W., Gelmann E. P. Loss of epithelial markers and acquisition of vimentin expression in adriamycin- and vinblastine-resistant human breast cancer cell lines. Cancer Res. 1992 Oct 1;52(19):5190–5197. [PubMed] [Google Scholar]
- Sommers C. L., Walker-Jones D., Heckford S. E., Worland P., Valverius E., Clark R., McCormick F., Stampfer M., Abularach S., Gelmann E. P. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989 Aug 1;49(15):4258–4263. [PubMed] [Google Scholar]
- Steinert P. M., Liem R. K. Intermediate filament dynamics. Cell. 1990 Feb 23;60(4):521–523. doi: 10.1016/0092-8674(90)90651-t. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stover D. M., Carey I., Garzon R. J., Zehner Z. E. A negative regulatory factor is missing in a human metastatic breast cancer cell line. Cancer Res. 1994 Jun 15;54(12):3092–3095. [PubMed] [Google Scholar]
- Streuli C. H., Bissell M. J. Mammary epithelial cells, extracellular matrix, and gene expression. Cancer Treat Res. 1991;53:365–381. doi: 10.1007/978-1-4615-3940-7_17. [DOI] [PubMed] [Google Scholar]
- Tawil N., Wilson P., Carbonetto S. Integrins in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993 Jan;120(1):261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Trevor K. T. Disruption of keratin filaments in embryonic epithelial cell types. New Biol. 1990 Nov;2(11):1004–1014. [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zutter M. M., Santoro S. A., Staatz W. D., Tsung Y. L. Re-expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci U S A. 1995 Aug 1;92(16):7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]