Abstract
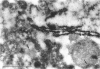
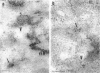
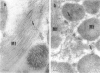
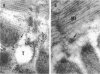
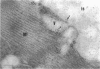
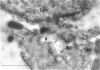
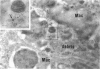
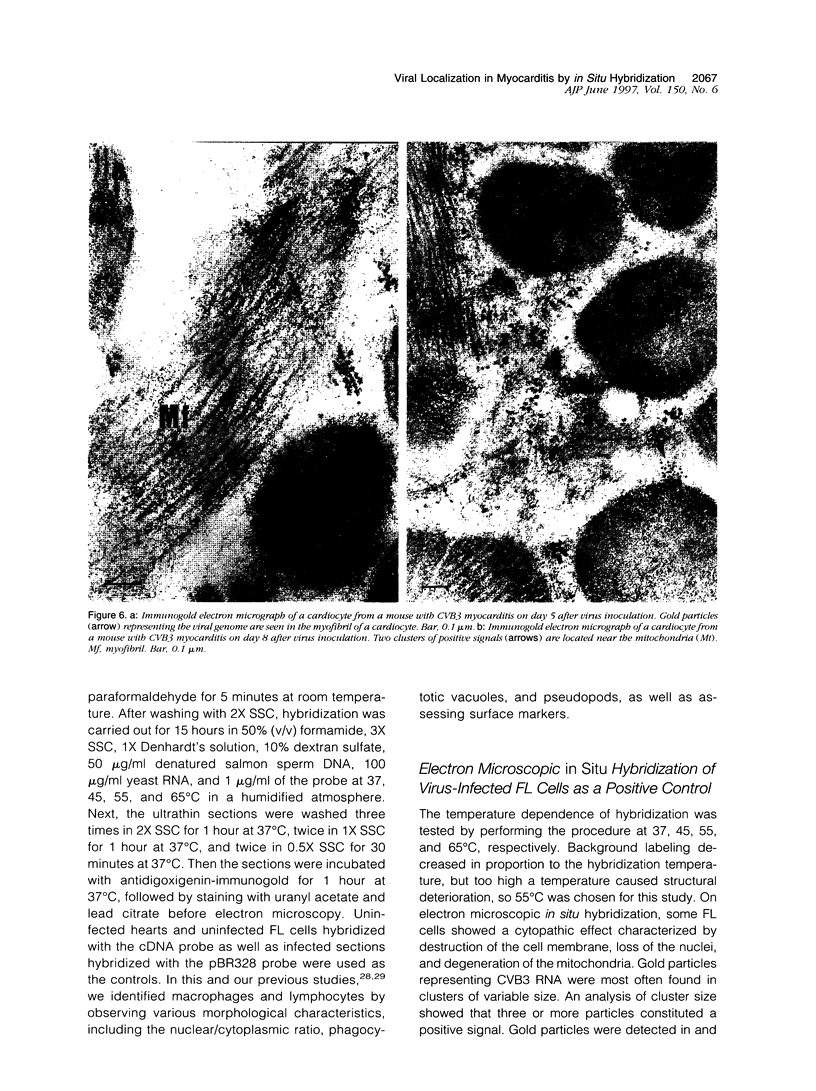
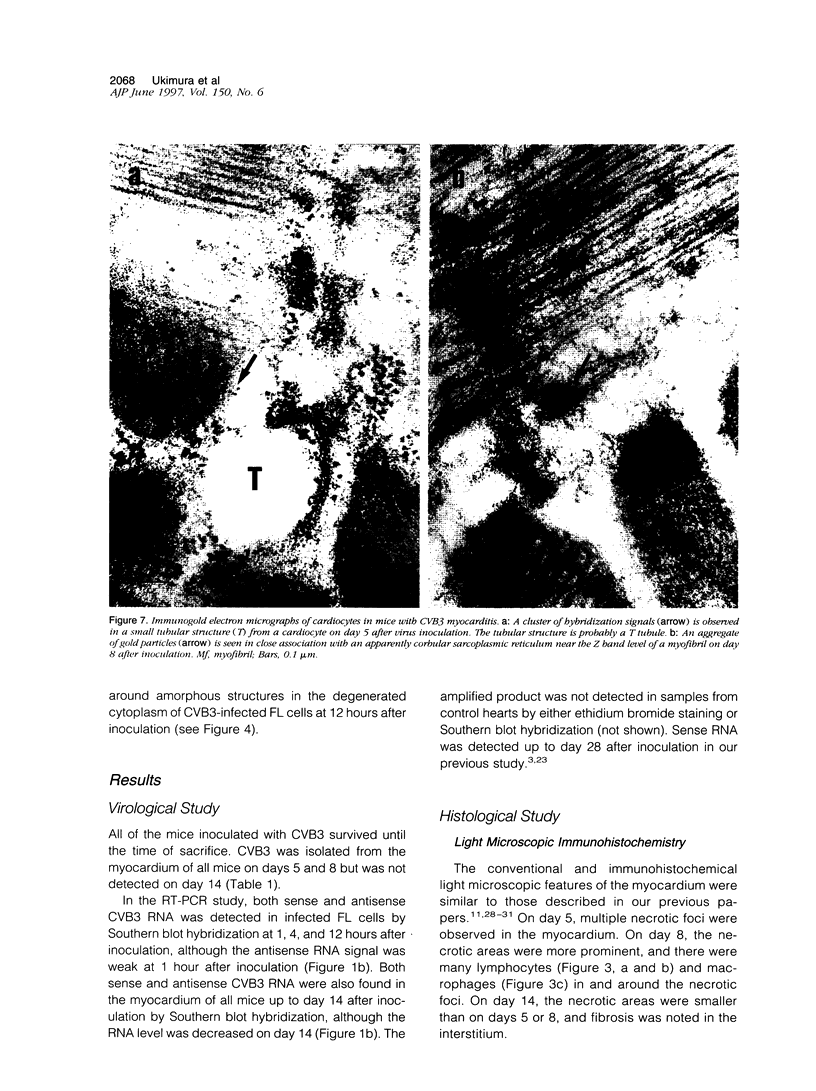
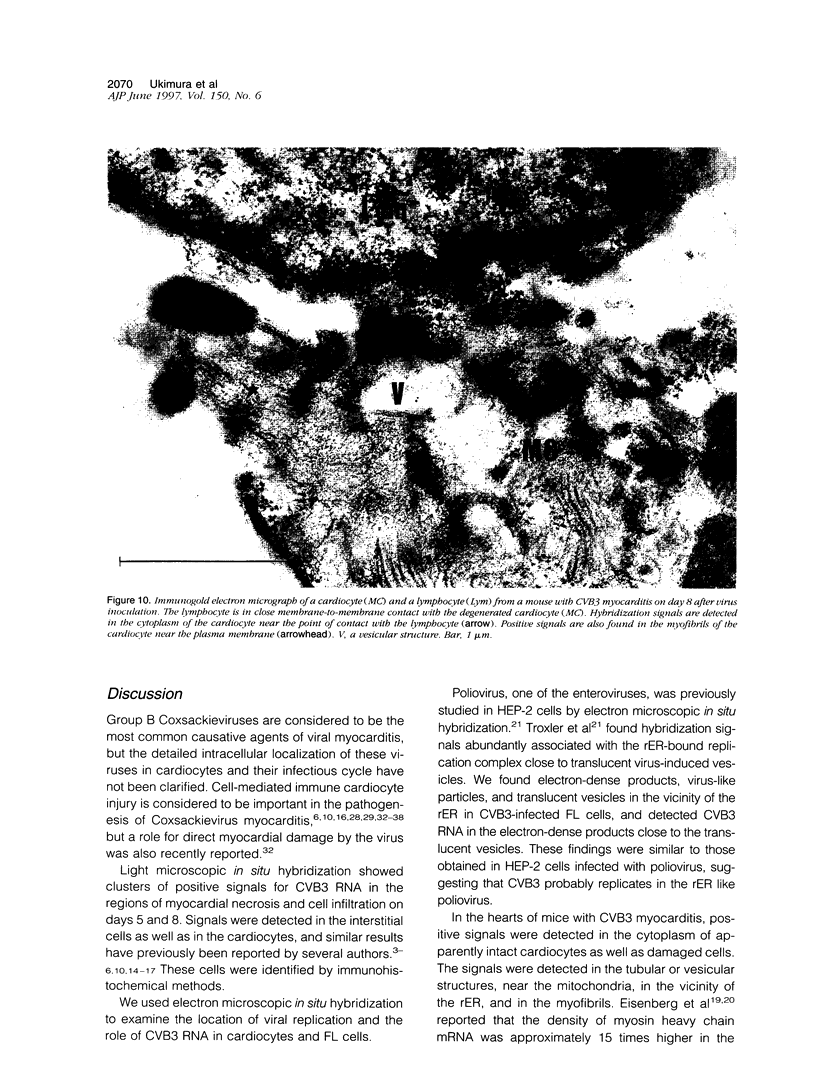
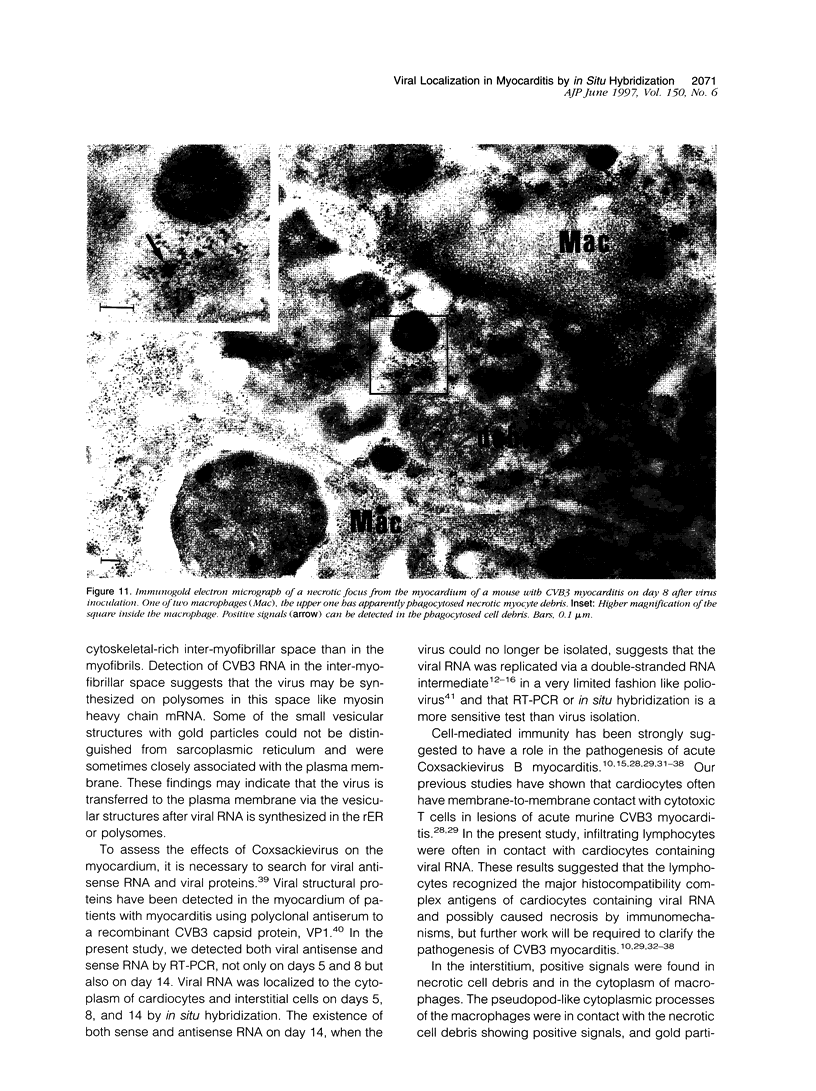
Group B Coxsackieviruses are a common cause of myocarditis. To detect the viral genome and its localization in the myocardium, we examined C3H/He mice with Coxsackievirus B3 (CVB3) myocarditis on days 5, 8, and 14 after inoculation by the reverse transcriptase polymerase chain reaction and by in situ hybridization. Sense and antisense CVB3 RNA were detected in the myocardium of all mice up to day 14 by reverse transcriptase polymerase chain reaction. Light microscopic in situ hybridization with a cDNA probe for CVB3 showed clusters of positive signals in the areas of myocardial necrosis and cell infiltration. With electron microscopic in situ hybridization, CVB3 RNA was detected in the cytoplasm of cardiocytes, between the myofibrils, near the mitochondria, and in tubular or vesicular structures. Viral RNA was also detected in necrotic debris, in the cytoplasm of macrophages, and in the cytoplasm of interstitial fibroblasts. These findings suggest that CVB3 RNA is replicated in the cytoplasm of cardiocytes, transferred into tubular or vesicular structures, released into the interstitium, and phagocytosed by macrophages. Some positive signals were also detected in the cytoplasm of cardiocytes showing close contact with infiltrating lymphocytes, suggesting that the lymphocytes recognized virus-infected cardiocytes and caused cell-mediated immune cardiocyte damage.
Full text
PDF
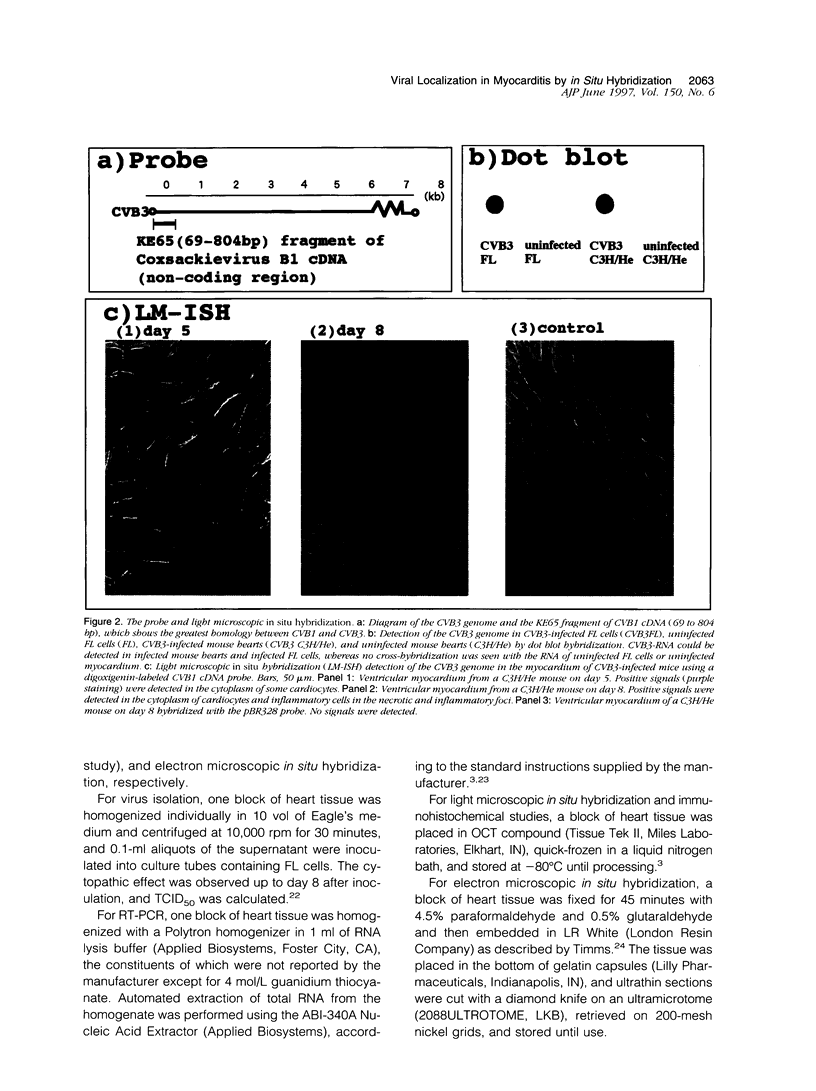
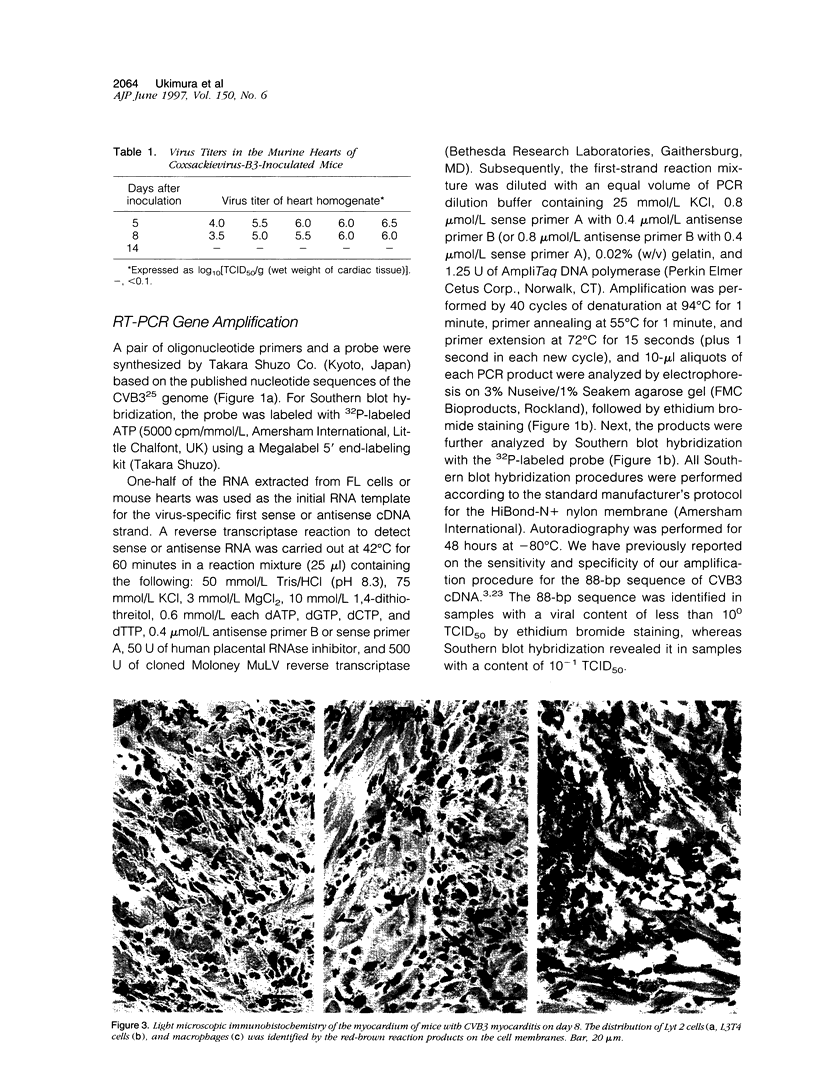
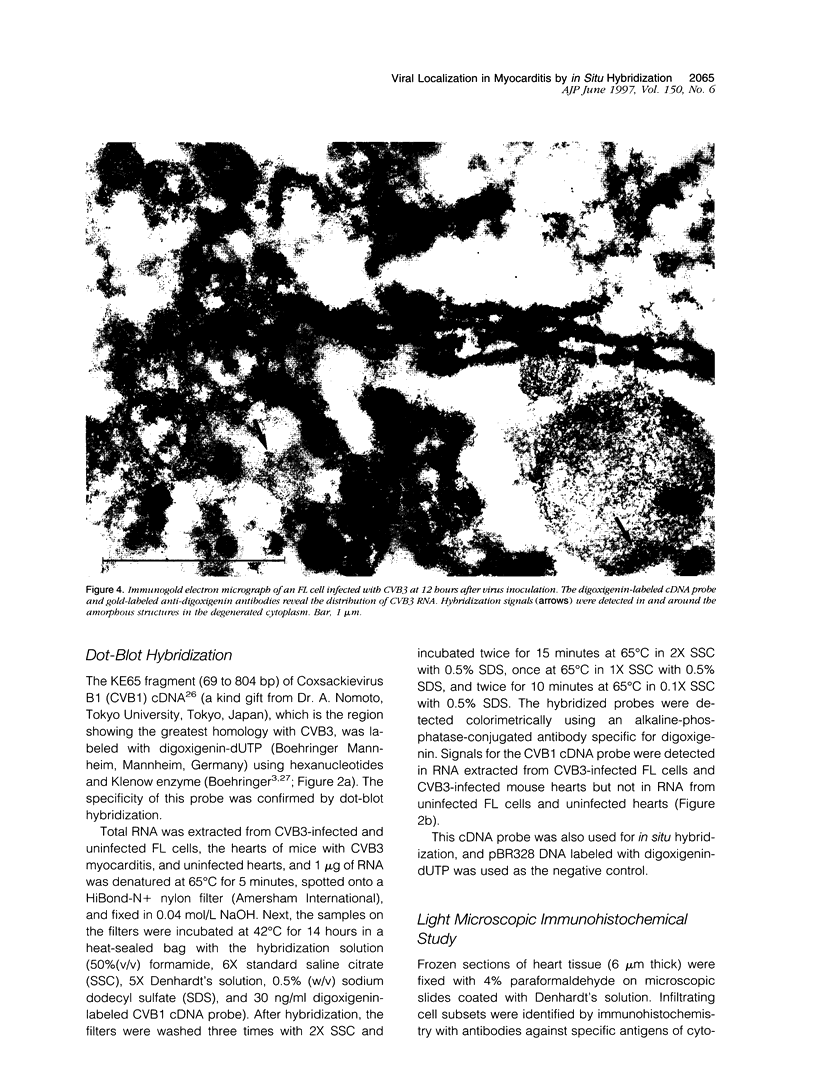
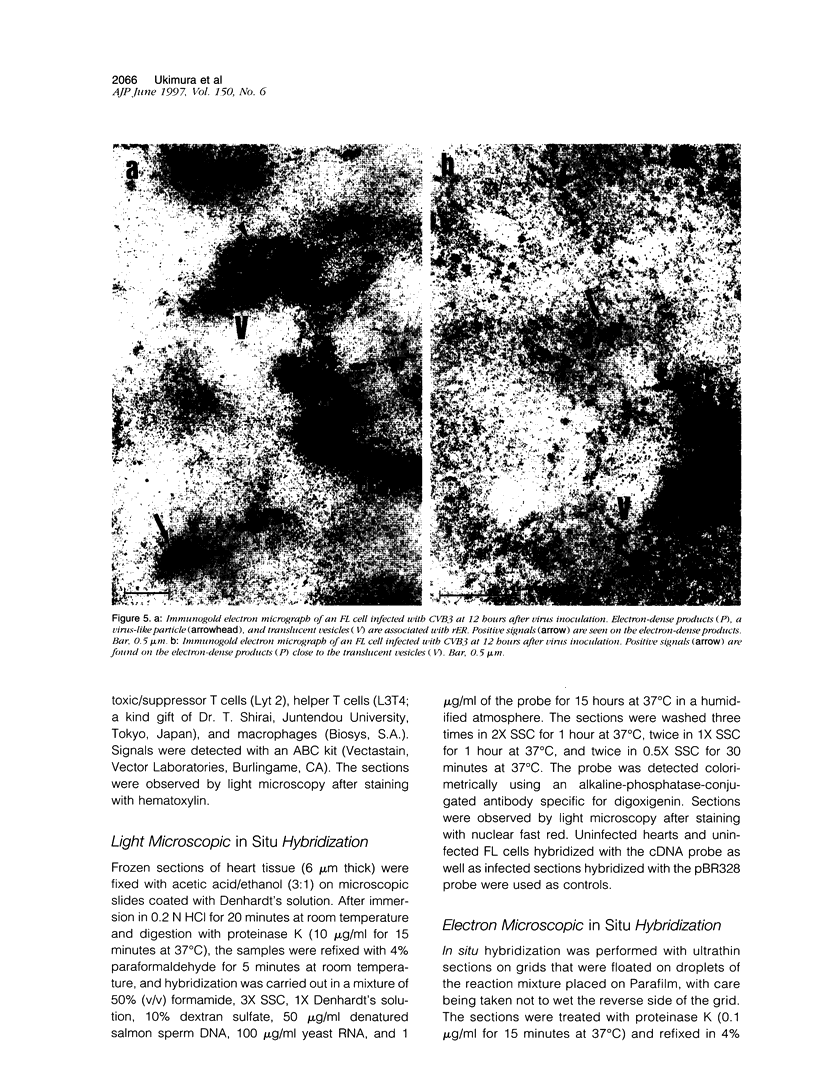




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Bowles N. E., Cunningham L., Freeke C. A., Olsen E. G., Rose M. L., Meany B., Why H. J., Richardson P. J. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur Heart J. 1991 Aug;12 (Suppl 500):56–59. doi: 10.1093/eurheartj/12.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P. Persistent infection of YAC-1 cells by coxsackievirus B3. J Gen Virol. 1988 Jan;69(Pt 1):59–65. doi: 10.1099/0022-1317-69-1-59. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Hayashi T., Kotaka M., Kawamura K. Cell-mediated immune cardiocyte injury in viral myocarditis of mice and patients. Jpn Circ J. 1989 Jan;53(1):61–77. doi: 10.1253/jcj.53.61. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Hayashi T., Kotaka M., Morita H., Kawamura K. Immunohistochemical study of the myocardium in murine Coxsackie B3 virus myocarditis using monoclonal antibodies--significance of Lyt 1 antigen-bearing lymphocytes in cell-mediated immunity. Jpn Circ J. 1986 Dec;50(12):1268–1274. doi: 10.1253/jcj.50.1268. [DOI] [PubMed] [Google Scholar]
- Deguchi H. Ultrastructural alterations of the myocardium in coxsackie B-3 virus myocarditis in mice. 18 months follow-up study by transmission and analytical electron microscopy. Jpn Circ J. 1981 Jun;45(6):695–712. doi: 10.1253/jcj.45.695. [DOI] [PubMed] [Google Scholar]
- Easton A. J., Eglin R. P. The detection of coxsackievirus RNA in cardiac tissue by in situ hybridization. J Gen Virol. 1988 Feb;69(Pt 2):285–291. doi: 10.1099/0022-1317-69-2-285. [DOI] [PubMed] [Google Scholar]
- Eisenberg B. R., Goldspink P. H., Wenderoth M. P. Distribution of myosin heavy chain mRNA in normal and hyperthyroid heart. J Mol Cell Cardiol. 1991 Mar;23(3):287–296. doi: 10.1016/0022-2828(91)90065-t. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., Farquharson M. A., Cameron S. O., McGill M., Schönke H., Kandolf R. A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia. 1990 May;33(5):290–298. doi: 10.1007/BF00403323. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Arizpe H. M., Higdon A. L., Wood H. J., Bowers D. F., Rozek M. M., Crawley R. Molecular mimicry, anti-coxsackievirus B3 neutralizing monoclonal antibodies, and myocarditis. J Immunol. 1995 Mar 15;154(6):2983–2995. [PubMed] [Google Scholar]
- Hofschneider P. H., Klingel K., Kandolf R. Toward understanding the pathogenesis of enterovirus-induced cardiomyopathy: molecular and ultrastructural approaches. J Struct Biol. 1990 Jul-Sep;104(1-3):32–37. doi: 10.1016/1047-8477(90)90054-g. [DOI] [PubMed] [Google Scholar]
- Hohenadl C., Klingel K., Mertsching J., Hofschneider P. H., Kandolf R. Strand-specific detection of enteroviral RNA in myocardial tissue by in situ hybridization. Mol Cell Probes. 1991 Feb;5(1):11–20. doi: 10.1016/0890-8508(91)90033-g. [DOI] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Klingel K., Zell R., Canu A., Fortmüller U., Hohenadl C., Albrecht M., Reimann B. Y., Franz W. M., Heim A. Molecular mechanisms in the pathogenesis of enteroviral heart disease: acute and persistent infections. Clin Immunol Immunopathol. 1993 Aug;68(2):153–158. doi: 10.1006/clin.1993.1112. [DOI] [PubMed] [Google Scholar]
- Koide H., Kitaura Y., Deguchi H., Ukimura A., Kawamura K., Hirai K. Genomic detection of enteroviruses in the myocardium--studies on animal hearts with coxsackievirus B3 myocarditis and endomyocardial biopsies from patients with myocarditis and dilated cardiomyopathy. Jpn Circ J. 1992 Oct;56(10):1081–1093. doi: 10.1253/jcj.56.1081. [DOI] [PubMed] [Google Scholar]
- Koide H., Kitaura Y., Deguchi H., Ukimura A., Kawamura K., Hirai K. Viral genomic detection in the hearts of C3H/He mice with experimental Coxsackievirus B3 myocarditis by gene amplification using the polymerase chain reaction. Jpn Circ J. 1992 Feb;56(2):148–156. doi: 10.1253/jcj.56.148. [DOI] [PubMed] [Google Scholar]
- Kuge S., Saito I., Nomoto A. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J Mol Biol. 1986 Dec 5;192(3):473–487. doi: 10.1016/0022-2836(86)90270-6. [DOI] [PubMed] [Google Scholar]
- Kyu B., Matsumori A., Sato Y., Okada I., Chapman N. M., Tracy S. Cardiac persistence of cardioviral RNA detected by polymerase chain reaction in a murine model of dilated cardiomyopathy. Circulation. 1992 Aug;86(2):522–530. doi: 10.1161/01.cir.86.2.522. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Martino T. A., Liu P., Sole M. J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res. 1994 Feb;74(2):182–188. doi: 10.1161/01.res.74.2.182. [DOI] [PubMed] [Google Scholar]
- Matteucci D., Paglianti M., Giangregorio A. M., Capobianchi M. R., Dianzani F., Bendinelli M. Group B coxsackieviruses readily establish persistent infections in human lymphoid cell lines. J Virol. 1985 Nov;56(2):651–654. doi: 10.1128/jvi.56.2.651-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus B. M., Chow L. H., Wilson J. E., Anderson D. R., Gulizia J. M., Gauntt C. J., Klingel K. E., Beisel K. W., Kandolf R. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993 Aug;68(2):159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- Morita H. Experimental coxsackie B-3 virus myocarditis in golden hamsters. Light and electron microscopy findings in a long-term follow-up study. Jpn Circ J. 1981 Jun;45(6):713–729. doi: 10.1253/jcj.45.713. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Arends M. J., Bishop P. E., Sizer K., Duvall E., Bird C. C. Sensitivity of digoxigenin and biotin labelled probes for detection of human papillomavirus by in situ hybridisation. J Clin Pathol. 1990 Oct;43(10):800–805. doi: 10.1136/jcp.43.10.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D. A., Lane J. R., Allen G. S., Herskowitz A., Rose N. R. Viral myocarditis leading to cardiomyopathy: do cytokines contribute to pathogenesis? Clin Immunol Immunopathol. 1993 Aug;68(2):181–190. doi: 10.1006/clin.1993.1116. [DOI] [PubMed] [Google Scholar]
- Neumann D. A., Rose N. R., Ansari A. A., Herskowitz A. Induction of multiple heart autoantibodies in mice with coxsackievirus B3- and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994 Jan 1;152(1):343–350. [PubMed] [Google Scholar]
- Rabausch-Starz I., Schwaiger A., Grünewald K., Müller-Hermelink H. K., Neu N. Persistence of virus and viral genome in myocardium after coxsackievirus B3-induced murine myocarditis. Clin Exp Immunol. 1994 Apr;96(1):69–74. doi: 10.1111/j.1365-2249.1994.tb06232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A. Autoimmunity in myocarditis: models and mechanisms. Clin Immunol Immunopathol. 1993 Aug;68(2):95–99. doi: 10.1006/clin.1993.1102. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A., Abzug M. J., Murray R. S., Murphy N. L., Levin M. J. Intracellular detection of sense and antisense enteroviral RNA by in situ hybridization. J Virol Methods. 1988 Dec;22(2-3):295–301. doi: 10.1016/0166-0934(88)90111-5. [DOI] [PubMed] [Google Scholar]
- Sato S., Tsutsumi R., Burke A., Carlson G., Porro V., Seko Y., Okumura K., Kawana R., Virmani R. Persistence of replicating coxsackievirus B3 in the athymic murine heart is associated with development of myocarditic lesions. J Gen Virol. 1994 Nov;75(Pt 11):2911–2924. doi: 10.1099/0022-1317-75-11-2911. [DOI] [PubMed] [Google Scholar]
- Schnurr D. P., Schmidt N. J. Persistent infection of mouse fibroblasts with Coxsackievirus. Arch Virol. 1984;81(1-2):91–101. doi: 10.1007/BF01309299. [DOI] [PubMed] [Google Scholar]
- Seko Y., Shinkai Y., Kawasaki A., Yagita H., Okumura K., Takaku F., Yazaki Y. Expression of perforin in infiltrating cells in murine hearts with acute myocarditis caused by coxsackievirus B3. Circulation. 1991 Aug;84(2):788–795. doi: 10.1161/01.cir.84.2.788. [DOI] [PubMed] [Google Scholar]
- Seko Y., Tsuchimochi H., Nakamura T., Okumura K., Naito S., Imataka K., Fujii J., Takaku F., Yazaki Y. Expression of major histocompatibility complex class I antigen in murine ventricular myocytes infected with Coxsackievirus B3. Circ Res. 1990 Aug;67(2):360–367. doi: 10.1161/01.res.67.2.360. [DOI] [PubMed] [Google Scholar]
- Timms B. G. Postembedding immunogold labeling for electron microscopy using "LR White" resin. Am J Anat. 1986 Feb-Mar;175(2-3):267–275. doi: 10.1002/aja.1001750211. [DOI] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., McManus B. M., Pallansch M. A., Beck M. A., Carstens J. A molecular and serologic evaluation of enteroviral involvement in human myocarditis. J Mol Cell Cardiol. 1990 Apr;22(4):403–414. doi: 10.1016/0022-2828(90)91476-n. [DOI] [PubMed] [Google Scholar]
- Troxler M., Egger D., Pfister T., Bienz K. Intracellular localization of poliovirus RNA by in situ hybridization at the ultrastructural level using single-stranded riboprobes. Virology. 1992 Dec;191(2):687–697. doi: 10.1016/0042-6822(92)90244-j. [DOI] [PubMed] [Google Scholar]
- Wenderoth M. P., Eisenberg B. R. Ultrastructural distribution of myosin heavy chain mRNA in cardiac tissue: a comparison of frozen and LR White embedment. J Histochem Cytochem. 1991 Aug;39(8):1025–1033. doi: 10.1177/39.8.1856452. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]