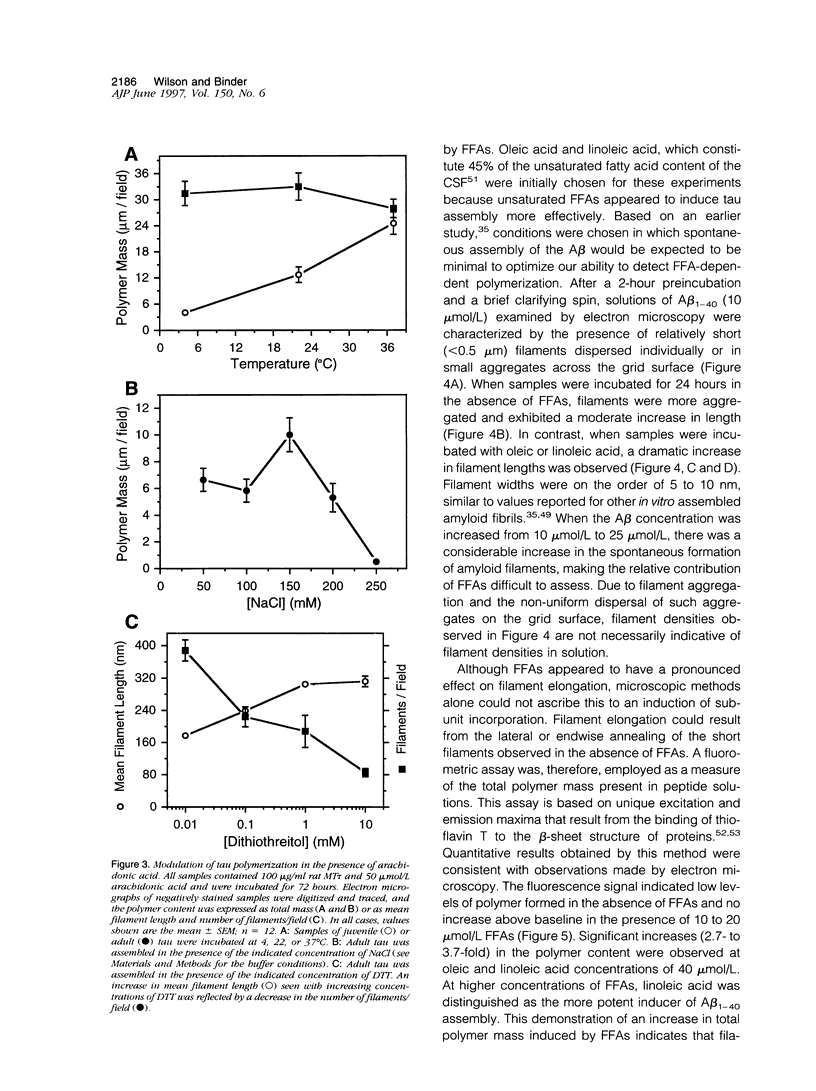
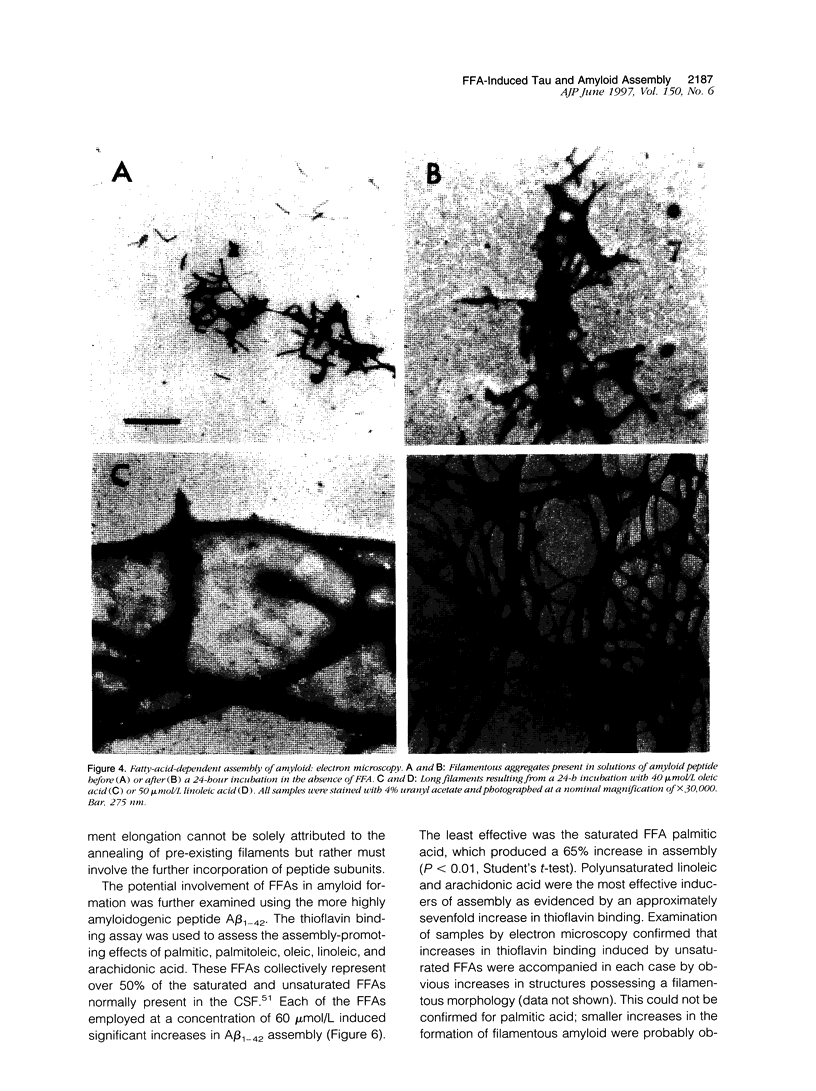
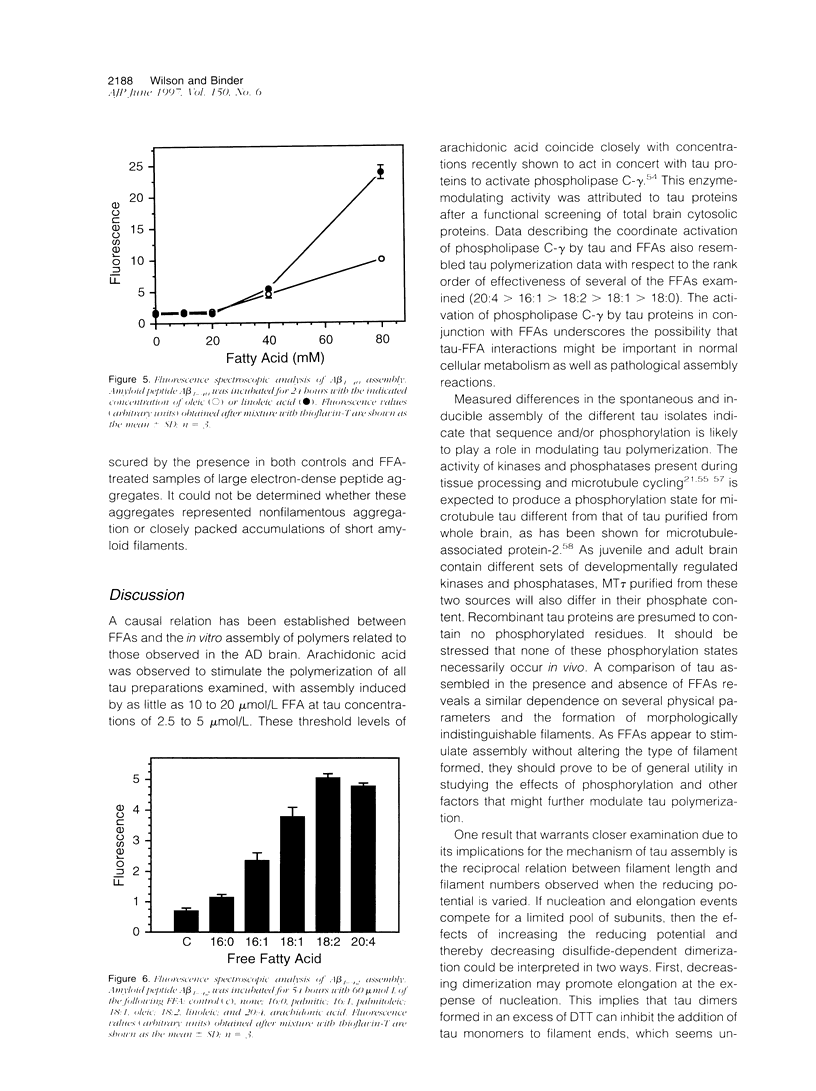
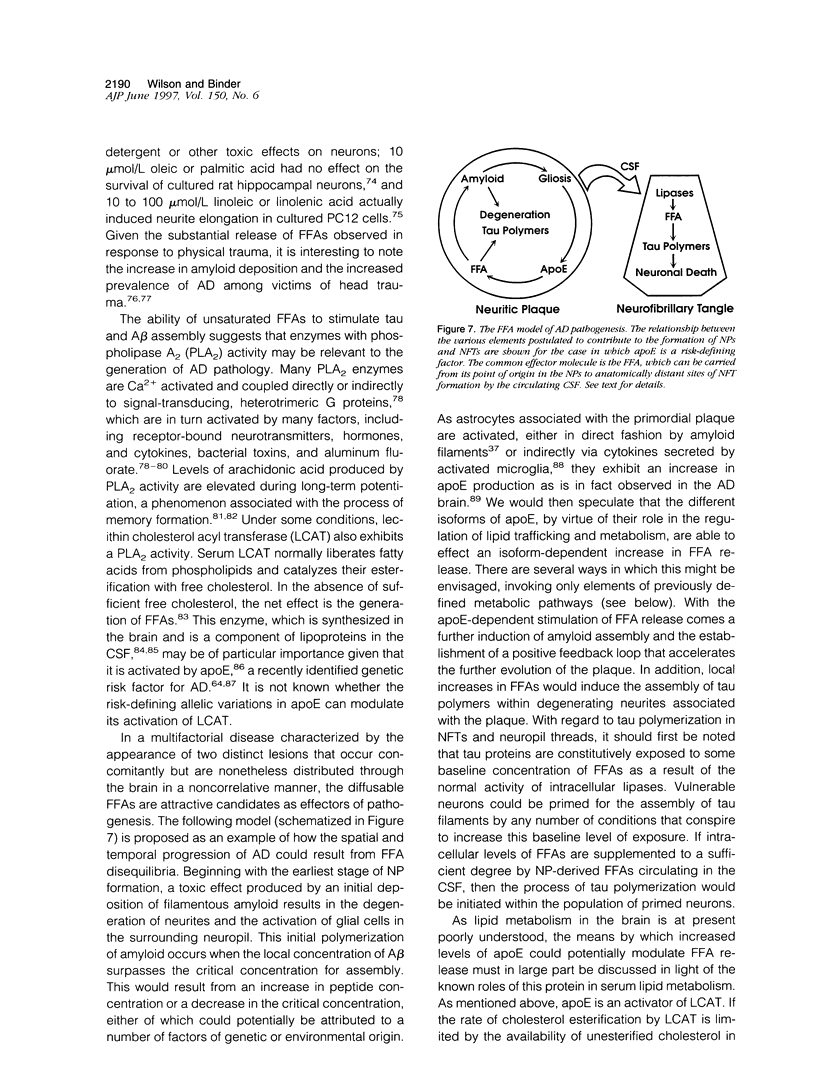
Abstract
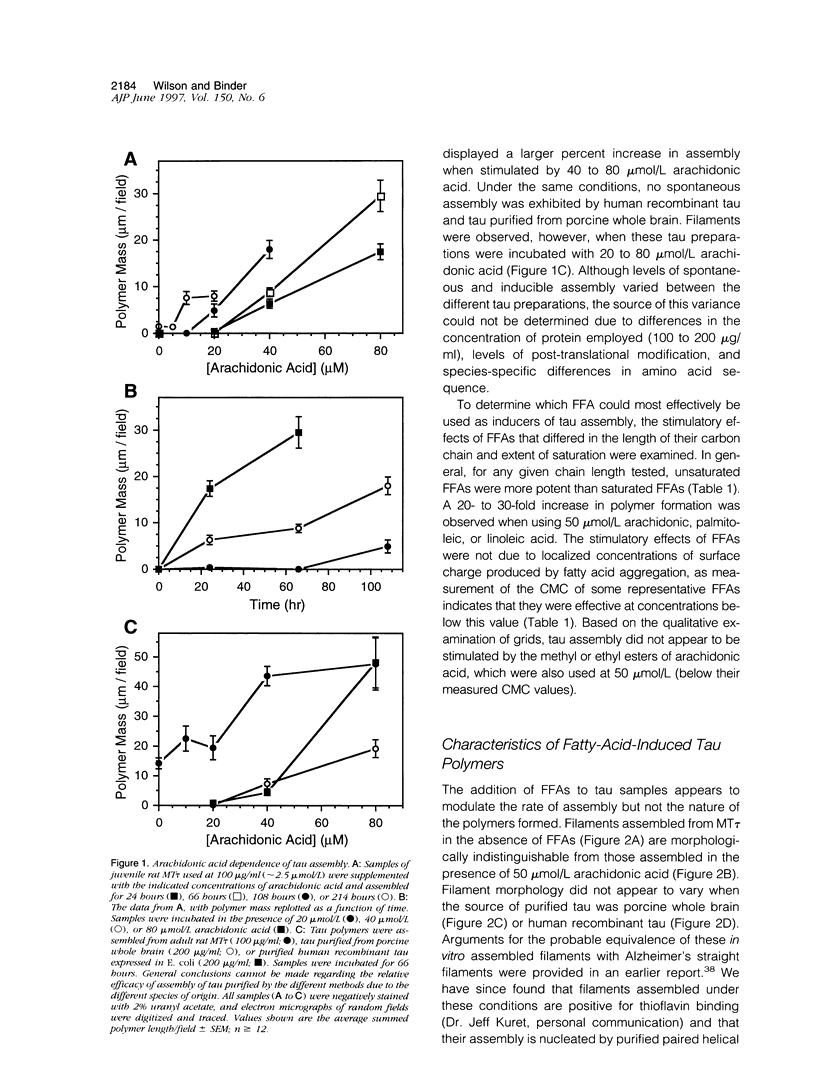
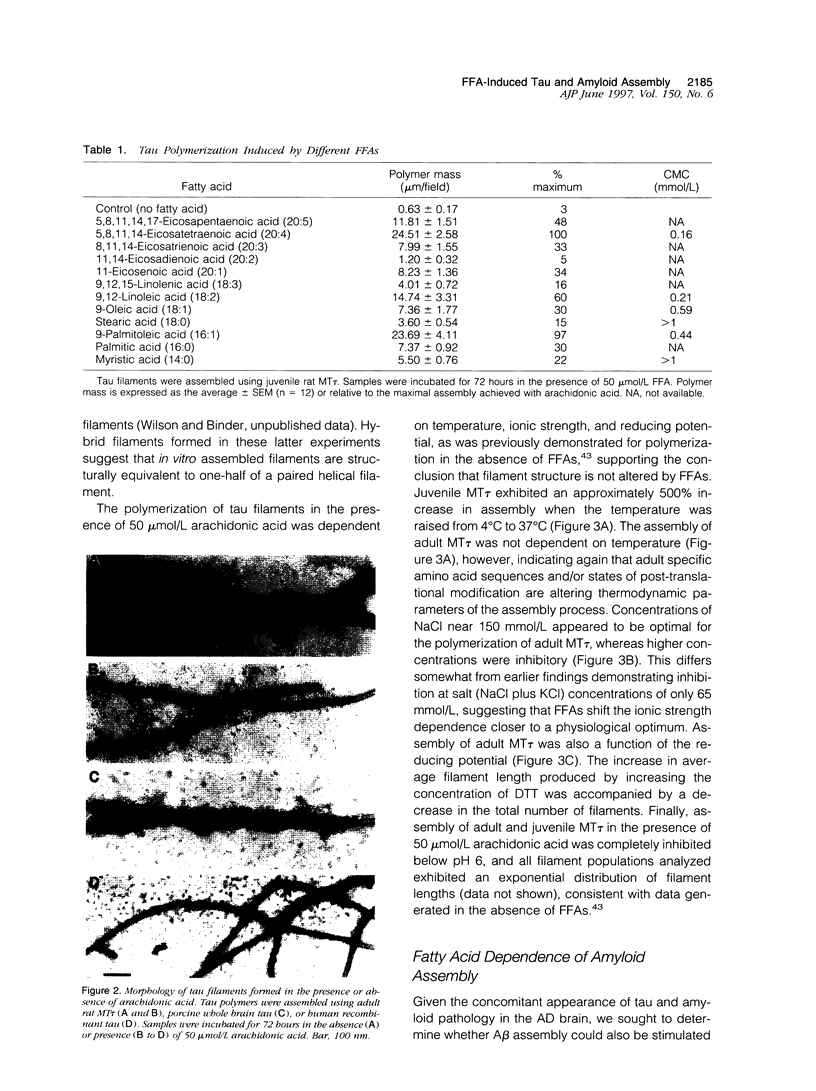
Alzheimer's disease is a degenerative disorder of the central nervous system, characterized by the concomitant deposition of extracellular filaments composed of beta-amyloid peptides and intracellular filaments composed of the microtubule-associated protein tau. We have discovered that free fatty acids (FFAs) stimulate the assembly of both amyloid and tau filaments in vitro. The minimal concentration of arachidonic acid observed to stimulate tau assembly ranged from 10 to 20 mumol/L, depending on the source of the purified tau. Tau preparations that do not exhibit spontaneous assembly were among those induced to polymerize by arachidonic acid. All long-chain FFAs tested enhanced assembly to some extent, although greater stimulation was usually associated with unsaturated forms. Utilizing fluorescence spectroscopy, unsaturated FFAs were also demonstrated to induce beta-amyloid assembly. The minimal concentration of oleic or linoleic acid observed to stimulate the assembly of amyloid was 40 mumol/L. The filamentous nature of these thioflavin-binding amyloid polymers was verified by electron microscopy. These data define a new set of tools for examining the polymerization of amyloid and tau proteins and suggest that cortical elevations of FFAs may constitute a unifying stimulatory event driving the formation of two of the obvious pathogenetic lesions in Alzheimer's disease.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold C. S., Johnson G. V., Cole R. N., Dong D. L., Lee M., Hart G. W. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J Biol Chem. 1996 Nov 15;271(46):28741–28744. doi: 10.1074/jbc.271.46.28741. [DOI] [PubMed] [Google Scholar]
- Aron L., Jones S., Fielding C. J. Human plasma lecithin-cholesterol acyltransferase. Characterization of cofactor-dependent phospholipase activity. J Biol Chem. 1978 Oct 25;253(20):7220–7226. [PubMed] [Google Scholar]
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Banaszak L., Winter N., Xu Z., Bernlohr D. A., Cowan S., Jones T. A. Lipid-binding proteins: a family of fatty acid and retinoid transport proteins. Adv Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- Berry J. F., Bovis M., Logothetis J. Determination of the fatty acid composition of cerebrospinal fluid by gas-liquid chromatography. Neurology. 1965 Dec;15(12):1089–1094. doi: 10.1212/wnl.15.12.1089. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Breitner J. C., Gau B. A., Welsh K. A., Plassman B. L., McDonald W. M., Helms M. J., Anthony J. C. Inverse association of anti-inflammatory treatments and Alzheimer's disease: initial results of a co-twin control study. Neurology. 1994 Feb;44(2):227–232. doi: 10.1212/wnl.44.2.227. [DOI] [PubMed] [Google Scholar]
- Burdick D., Soreghan B., Kwon M., Kosmoski J., Knauer M., Henschen A., Yates J., Cotman C., Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/beta amyloid peptide analogs. J Biol Chem. 1992 Jan 5;267(1):546–554. [PubMed] [Google Scholar]
- Carmel G., Leichus B., Cheng X., Patterson S. D., Mirza U., Chait B. T., Kuret J. Expression, purification, crystallization, and preliminary x-ray analysis of casein kinase-1 from Schizosaccharomyces pombe. J Biol Chem. 1994 Mar 11;269(10):7304–7309. [PubMed] [Google Scholar]
- Castano E. M., Prelli F., Wisniewski T., Golabek A., Kumar R. A., Soto C., Frangione B. Fibrillogenesis in Alzheimer's disease of amyloid beta peptides and apolipoprotein E. Biochem J. 1995 Mar 1;306(Pt 2):599–604. doi: 10.1042/bj3060599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Clements M. P., Bliss T. V., Lynch M. A. Increase in arachidonic acid concentration in a postsynaptic membrane fraction following the induction of long-term potentiation in the dentate gyrus. Neuroscience. 1991;45(2):379–389. doi: 10.1016/0306-4522(91)90235-g. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Dehaut F., Bertrand I., Miltaud T., Pouplard-Barthelaix A., Maingault M. n-6 polyunsaturated fatty acids increase the neurite length of PC12 cells and embryonic chick motoneurons. Neurosci Lett. 1993 Oct 29;161(2):133–136. doi: 10.1016/0304-3940(93)90277-r. [DOI] [PubMed] [Google Scholar]
- Diedrich J. F., Minnigan H., Carp R. I., Whitaker J. N., Race R., Frey W., 2nd, Haase A. T. Neuropathological changes in scrapie and Alzheimer's disease are associated with increased expression of apolipoprotein E and cathepsin D in astrocytes. J Virol. 1991 Sep;65(9):4759–4768. doi: 10.1128/jvi.65.9.4759-4768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin D. G., Kirschner M. W. Tau protein function in living cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías G. A., Vial C., Maccioni R. B. Functional domains on chemically modified tau protein. Cell Mol Neurobiol. 1993 Apr;13(2):173–182. doi: 10.1007/BF00735373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez E., Pallini R., Bracali A. M., Perilli V., Perotti V., Pelosi G. Early changes in cerebrospinal fluid Ca2+ and FFA levels following experimental spinal cord injury. Neurol Res. 1988 Jun;10(2):66–68. doi: 10.1080/01616412.1988.11739817. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Spillantini M. G., Crowther R. A., Cohen P., Vanmechelen E., Probst A., Götz J., Bürki K. Tau protein in Alzheimer's disease. Biochem Soc Trans. 1995 Feb;23(1):80–85. doi: 10.1042/bst0230080. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hardy J. A., Higgins G. A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992 Apr 10;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M., Kurland L. T., Zimmerman H. M. The fine structure of some intraganglionic alterations. Neurofibrillary tangles, granulovacuolar bodies and "rod-like" structures as seen in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J Neuropathol Exp Neurol. 1968 Apr;27(2):167–182. [PubMed] [Google Scholar]
- Hirokawa N., Shiomura Y., Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988 Oct;107(4):1449–1459. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. C., Jhon D. Y., Bae Y. S., Kim J. H., Rhee S. G. Activation of phospholipase C-gamma by the concerted action of tau proteins and arachidonic acid. J Biol Chem. 1996 Aug 2;271(31):18342–18349. doi: 10.1074/jbc.271.31.18342. [DOI] [PubMed] [Google Scholar]
- Jarrett J. T., Berger E. P., Lansbury P. T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer's disease. Biochemistry. 1993 May 11;32(18):4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- KIDD M. Paired helical filaments in electron microscopy of Alzheimer's disease. Nature. 1963 Jan 12;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Kenessey A., Yen S. H., Liu W. K., Yang X. R., Dunlop D. S. Detection of D-aspartate in tau proteins associated with Alzheimer paired helical filaments. Brain Res. 1995 Mar 27;675(1-2):183–189. doi: 10.1016/0006-8993(95)00061-t. [DOI] [PubMed] [Google Scholar]
- Kondo J., Honda T., Mori H., Hamada Y., Miura R., Ogawara M., Ihara Y. The carboxyl third of tau is tightly bound to paired helical filaments. Neuron. 1988 Nov;1(9):827–834. doi: 10.1016/0896-6273(88)90130-4. [DOI] [PubMed] [Google Scholar]
- Kovatchev S., Vaz W. L., Eibl H. Lipid dependence of the membrane-bound D-lactate dehydrogenase of Escherichia coli. J Biol Chem. 1981 Oct 25;256(20):10369–10374. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993 Mar;2(3):404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma M. D., Bonay P., Colaço C., Avila J. Analysis of microtubule-associated protein tau glycation in paired helical filaments. J Biol Chem. 1994 Aug 26;269(34):21614–21619. [PubMed] [Google Scholar]
- Lomakin A., Chung D. S., Benedek G. B., Kirschner D. A., Teplow D. B. On the nucleation and growth of amyloid beta-protein fibrils: detection of nuclei and quantitation of rate constants. Proc Natl Acad Sci U S A. 1996 Feb 6;93(3):1125–1129. doi: 10.1073/pnas.93.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q., Soria J. P., Wood J. G. p44mpk MAP kinase induces Alzheimer type alterations in tau function and in primary hippocampal neurons. J Neurosci Res. 1993 Jul 1;35(4):439–444. doi: 10.1002/jnr.490350411. [DOI] [PubMed] [Google Scholar]
- Lue L. F., Brachova L., Civin W. H., Rogers J. Inflammation, A beta deposition, and neurofibrillary tangle formation as correlates of Alzheimer's disease neurodegeneration. J Neuropathol Exp Neurol. 1996 Oct;55(10):1083–1088. [PubMed] [Google Scholar]
- Lynch M. A., Clements M. P., Voss K. L., Bramham C. R., Bliss T. V. Is arachidonic acid a retrograde messenger in long-term potentiation? Biochem Soc Trans. 1991 Apr;19(2):391–396. doi: 10.1042/bst0190391. [DOI] [PubMed] [Google Scholar]
- Maggio J. E., Stimson E. R., Ghilardi J. R., Allen C. J., Dahl C. E., Whitcomb D. C., Vigna S. R., Vinters H. V., Labenski M. E., Mantyh P. W. Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5462–5466. doi: 10.1073/pnas.89.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Shoemaker W. J., Shajenko L., Chambers T. E., Herbette L. G. Evidence for changes in the Alzheimer's disease brain cortical membrane structure mediated by cholesterol. Neurobiol Aging. 1992 May-Jun;13(3):413–419. doi: 10.1016/0197-4580(92)90116-f. [DOI] [PubMed] [Google Scholar]
- Matsuo E. S., Shin R. W., Billingsley M. L., Van deVoorde A., O'Connor M., Trojanowski J. Q., Lee V. M. Biopsy-derived adult human brain tau is phosphorylated at many of the same sites as Alzheimer's disease paired helical filament tau. Neuron. 1994 Oct;13(4):989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- McKee A. C., Kosik K. S., Kowall N. W. Neuritic pathology and dementia in Alzheimer's disease. Ann Neurol. 1991 Aug;30(2):156–165. doi: 10.1002/ana.410300206. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Papayannopoulos I. A., Styles J., Bobin S. A., Lin Y. Y., Biemann K., Iqbal K. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer's disease. Arch Biochem Biophys. 1993 Feb 15;301(1):41–52. doi: 10.1006/abbi.1993.1112. [DOI] [PubMed] [Google Scholar]
- Mori H., Takio K., Ogawara M., Selkoe D. J. Mass spectrometry of purified amyloid beta protein in Alzheimer's disease. J Biol Chem. 1992 Aug 25;267(24):17082–17086. [PubMed] [Google Scholar]
- Mortimer J. A., van Duijn C. M., Chandra V., Fratiglioni L., Graves A. B., Heyman A., Jorm A. F., Kokmen E., Kondo K., Rocca W. A. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20 (Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- Naiki H., Higuchi K., Hosokawa M., Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989 Mar;177(2):244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Okuda S., Saito H., Katsuki H. Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience. 1994 Dec;63(3):691–699. doi: 10.1016/0306-4522(94)90515-0. [DOI] [PubMed] [Google Scholar]
- Okuizumi K., Onodera O., Namba Y., Ikeda K., Yamamoto T., Seki K., Ueki A., Nanko S., Tanaka H., Takahashi H. Genetic association of the very low density lipoprotein (VLDL) receptor gene with sporadic Alzheimer's disease. Nat Genet. 1995 Oct;11(2):207–209. doi: 10.1038/ng1095-207. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Cummings B. J., Monzavi R., Cotman C. W. Beta-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer's disease. Neuroscience. 1994 Nov;63(2):517–531. doi: 10.1016/0306-4522(94)90547-9. [DOI] [PubMed] [Google Scholar]
- Pike C. J., Walencewicz-Wasserman A. J., Kosmoski J., Cribbs D. H., Glabe C. G., Cotman C. W. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995 Jan;64(1):253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- Probst A., Basler V., Bron B., Ulrich J. Neuritic plaques in senile dementia of Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res. 1983 Jun 6;268(2):249–254. doi: 10.1016/0006-8993(83)90490-0. [DOI] [PubMed] [Google Scholar]
- ROWE C. E. THE OCCURRENCE AND METABOLISM IN VITRO OF UNESTERIFIED FATTY ACID IN MOUSE BRAIN. Biochim Biophys Acta. 1964 Aug 5;84:424–434. doi: 10.1016/0926-6542(64)90006-x. [DOI] [PubMed] [Google Scholar]
- Risnik V. V., Adám G., Gusev N. B., Friedrich P. Casein kinases I and II bound to pig brain microtubules. Cell Mol Neurobiol. 1988 Sep;8(3):315–324. doi: 10.1007/BF00711173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. W., Gentleman S. M., Lynch A., Murray L., Landon M., Graham D. I. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994 Apr;57(4):419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Kirby L. C., Hempelman S. R., Berry D. L., McGeer P. L., Kaszniak A. W., Zalinski J., Cofield M., Mansukhani L., Willson P. Clinical trial of indomethacin in Alzheimer's disease. Neurology. 1993 Aug;43(8):1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- Sanan D. A., Weisgraber K. H., Russell S. J., Mahley R. W., Huang D., Saunders A., Schmechel D., Wisniewski T., Frangione B., Roses A. D. Apolipoprotein E associates with beta amyloid peptide of Alzheimer's disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Invest. 1994 Aug;94(2):860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996 Aug;2(8):864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- Schweers O., Mandelkow E. M., Biernat J., Mandelkow E. Oxidation of cysteine-322 in the repeat domain of microtubule-associated protein tau controls the in vitro assembly of paired helical filaments. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Simmons L. K., May P. C., Tomaselli K. J., Rydel R. E., Fuson K. S., Brigham E. F., Wright S., Lieberburg I., Becker G. W., Brems D. N. Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994 Mar;45(3):373–379. [PubMed] [Google Scholar]
- Smith K. M., Lawn R. M., Wilcox J. N. Cellular localization of apolipoprotein D and lecithin:cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipid Res. 1990 Jun;31(6):995–1004. [PubMed] [Google Scholar]
- Spector A. A., Fletcher J. E., Ashbrook J. D. Analysis of long-chain free fatty acid binding to bovine serum albumin by determination of stepwise equilibrium constants. Biochemistry. 1971 Aug 17;10(17):3229–3232. doi: 10.1021/bi00793a011. [DOI] [PubMed] [Google Scholar]
- Spector A. A., John K., Fletcher J. E. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969 Jan;10(1):56–67. [PubMed] [Google Scholar]
- Strittmatter W. J., Saunders A. M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G. S., Roses A. D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Weisgraber K. H., Huang D. Y., Dong L. M., Salvesen G. S., Pericak-Vance M., Schmechel D., Saunders A. M., Goldgaber D., Roses A. D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum I. The epidermal growth factor receptor is coupled to a phospholipase A2-specific pertussis toxin-inhibitable guanine nucleotide-binding regulatory protein in cultured rat inner medullary collecting tubule cells. J Biol Chem. 1990 Mar 15;265(8):4218–4222. [PubMed] [Google Scholar]
- Tellez-Nagel I., Wiśniewski H. M. Ultrastructure of neurofibrillary tangles in Steele-Richardson-Olszewski syndrome. Arch Neurol. 1973 Nov;29(5):324–327. doi: 10.1001/archneur.1973.00490290064007. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Lee V. M. Paired helical filament tau in Alzheimer's disease. The kinase connection. Am J Pathol. 1994 Mar;144(3):449–453. [PMC free article] [PubMed] [Google Scholar]
- Troncoso J. C., Costello A., Watson A. L., Jr, Johnson G. V. In vitro polymerization of oxidized tau into filaments. Brain Res. 1993 Jun 11;613(2):313–316. doi: 10.1016/0006-8993(93)90918-d. [DOI] [PubMed] [Google Scholar]
- Tsuyama S., Bramblett G. T., Huang K. P., Flavin M. Calcium/phospholipid-dependent kinase recognizes sites in microtubule-associated protein 2 which are phosphorylated in living brain and are not accessible to other kinases. J Biol Chem. 1986 Mar 25;261(9):4110–4116. [PubMed] [Google Scholar]
- Vallano M. L., Goldenring J. R., Buckholz T. M., Larson R. E., DeLorenzo R. J. Separation of endogenous calmodulin- and cAMP-dependent kinases from microtubule preparations. Proc Natl Acad Sci U S A. 1985 May;82(10):3202–3206. doi: 10.1073/pnas.82.10.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Z., Grundke-Iqbal I., Iqbal K. Glycosylation of microtubule-associated protein tau: an abnormal posttranslational modification in Alzheimer's disease. Nat Med. 1996 Aug;2(8):871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Langner C. A., Gordon J. I., Taylor B. A., McLean J. W., Lusis A. J. Tissue-specific expression, developmental regulation, and chromosomal mapping of the lecithin: cholesterol acyltransferase gene. Evidence for expression in brain and testes as well as liver. J Biol Chem. 1989 Dec 25;264(36):21573–21581. [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Wille H., Drewes G., Biernat J., Mandelkow E. M., Mandelkow E. Alzheimer-like paired helical filaments and antiparallel dimers formed from microtubule-associated protein tau in vitro. J Cell Biol. 1992 Aug;118(3):573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. M., Binder L. I. Polymerization of microtubule-associated protein tau under near-physiological conditions. J Biol Chem. 1995 Oct 13;270(41):24306–24314. doi: 10.1074/jbc.270.41.24306. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Thøgersen H. C., Edwards P. C., Runswick M. J., Jakes R., Walker J. E., Milstein C., Roth M., Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski K., Jervis G. A., Moretz R. C., Wisniewski H. M. Alzheimer neurofibrillary tangles in diseases other than senile and presenile dementia. Ann Neurol. 1979 Mar;5(3):288–294. doi: 10.1002/ana.410050311. [DOI] [PubMed] [Google Scholar]
- Yagishita S., Itoh Y., Nan W., Amano N. Reappraisal of the fine structure of Alzheimer's neurofibrillary tangles. Acta Neuropathol. 1981;54(3):239–246. doi: 10.1007/BF00687747. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Nakazato Y., Shoji M., Ihara Y., Hirai S. Ultrastructure of the neuropil threads in the Alzheimer brain: their dendritic origin and accumulation in the senile plaques. Acta Neuropathol. 1990;80(4):368–374. doi: 10.1007/BF00307689. [DOI] [PubMed] [Google Scholar]
- Yan S. D., Chen X., Schmidt A. M., Brett J., Godman G., Zou Y. S., Scott C. W., Caputo C., Frappier T., Smith M. A. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younkin S. G. The amyloid beta protein precursor mutations linked to familial Alzheimer's disease alter processing in a way that fosters amyloid deposition. Tohoku J Exp Med. 1994 Nov;174(3):217–223. doi: 10.1620/tjem.174.217. [DOI] [PubMed] [Google Scholar]
- Zorich N., Jonas A., Pownall H. J. Activation of lecithin cholesterol acyltransferase by human apolipoprotein E in discoidal complexes with lipids. J Biol Chem. 1985 Jul 25;260(15):8831–8837. [PubMed] [Google Scholar]