Abstract
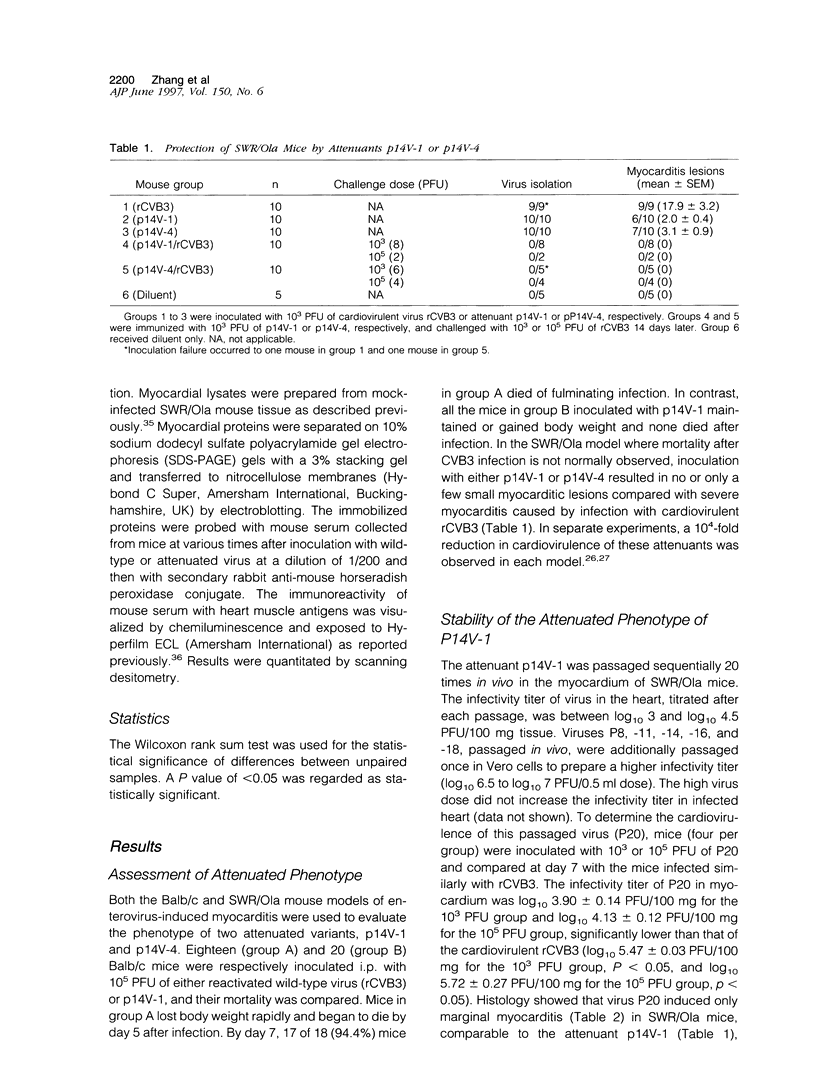
Coxsackievirus B3 (CVB3) is the enterovirus most frequently involved in human myocarditis or dilated cardiomyopathy. Attenuated variants were derived from a cardiovirulent CVB3 reactivated from a sequenced, full-length cDNA clone. The prophylactic potential of these variants was assessed in SWR/Ola (H-2q) mice. Animals immunized with attenuated variants of CVB3 were protected from myocarditis when challenged subsequently with the cardiovirulent wild-type virus. In contrast to nonimmunized controls, the wild-type virus was not isolated from myocardium of protected mice, nor was viral RNA detected in myocardium by reverse transcription nested polymerase chain reaction. Specific antibody to CVB3 was demonstrated by virus neutralization assay and by indirect immunofluorescence. The attenuated phenotype of one variant, p14V-1, remained stable throughout 20 consecutive passages in SWR mice and induced a markedly lower level of autoantibody against mouse cardiac myosin heavy chain than the cardiovirulent wild type. These data demonstrate that attenuated strains protect against CVB3-induced myocarditis in mice, that the attenuated phenotype is stable, and that they do not persist in myocardium nor induce a significant level of anti-heart anti-body against myosin heavy chain. These attenuants may be the basis of a live vaccine against CVB3 in the prevention of enteroviral heart muscle disease.
Full text
PDF


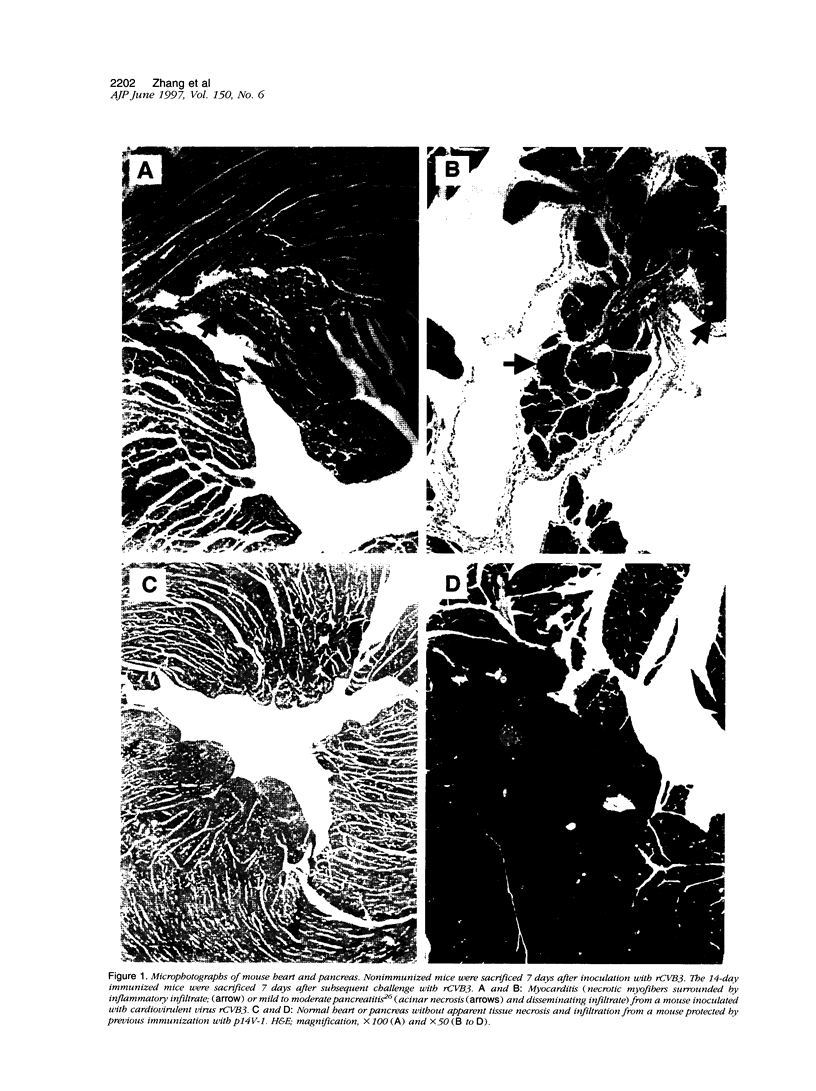
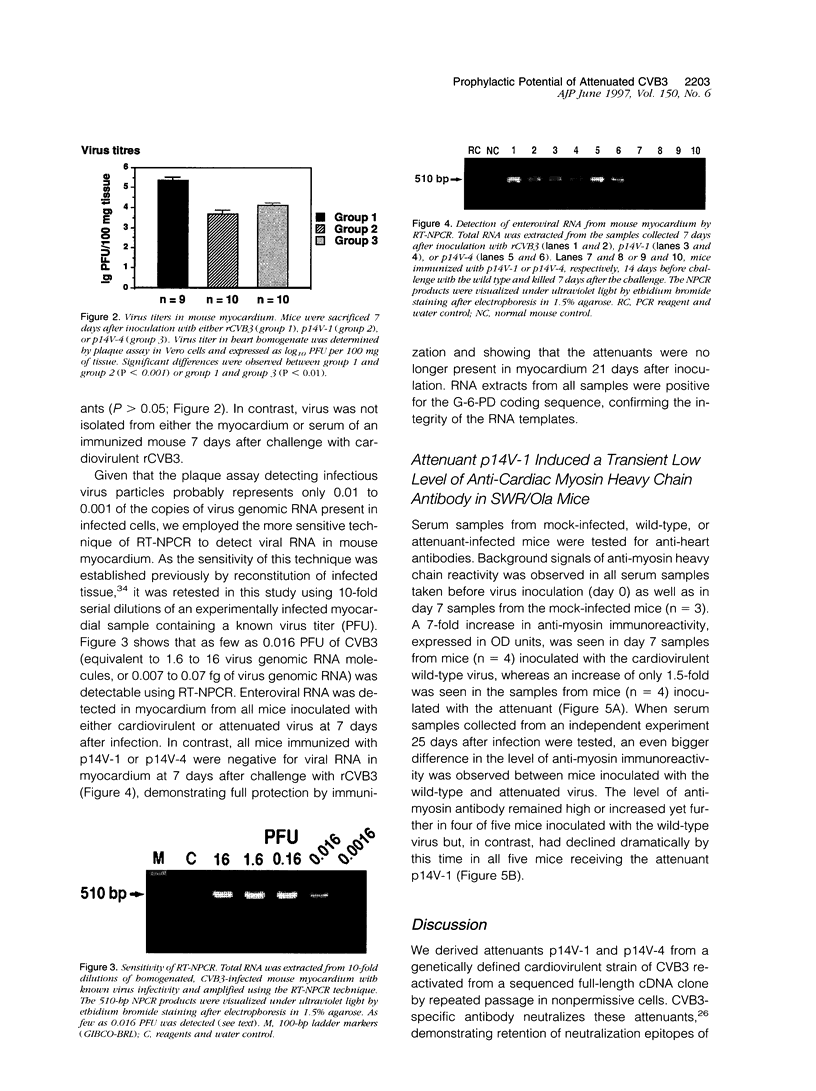
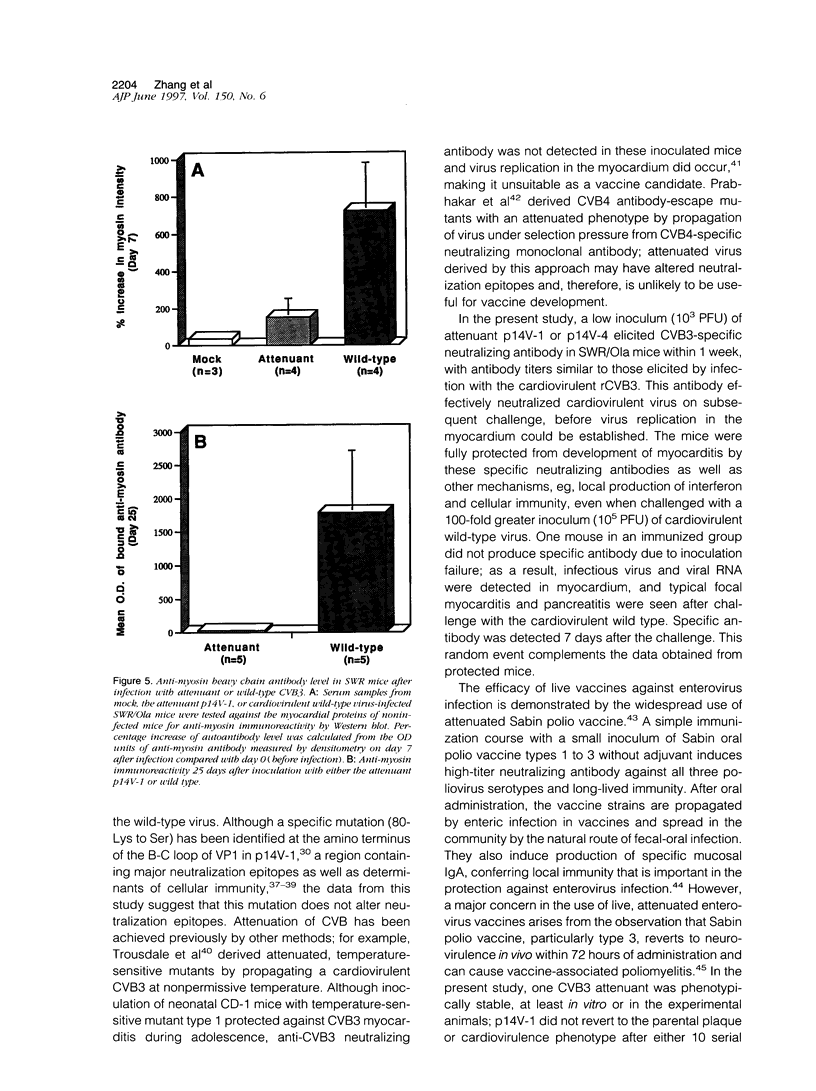



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoletti L., Hober D., Decoene C., Copin M. C., Lobert P. E., Dewilde A., Stankowiac C., Wattre P. Detection of enteroviral RNA by polymerase chain reaction in endomyocardial tissue of patients with chronic cardiac diseases. J Med Virol. 1996 Jan;48(1):53–59. doi: 10.1002/(SICI)1096-9071(199601)48:1<53::AID-JMV9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Archard L. C., Bowles N. E., Cunningham L., Freeke C. A., Olsen E. G., Rose M. L., Meany B., Why H. J., Richardson P. J. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur Heart J. 1991 Aug;12 (Suppl 500):56–59. doi: 10.1093/eurheartj/12.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- Boehm T., Spillantini M. G., Sofroniew M. V., Surani M. A., Rabbitts T. H. Developmentally regulated and tissue specific expression of mRNAs encoding the two alternative forms of the LIM domain oncogene rhombotin: evidence for thymus expression. Oncogene. 1991 May;6(5):695–703. [PubMed] [Google Scholar]
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Rose M. L., Taylor P., Banner N. R., Morgan-Capner P., Cunningham L., Archard L. C., Yacoub M. H. End-stage dilated cardiomyopathy. Persistence of enterovirus RNA in myocardium at cardiac transplantation and lack of immune response. Circulation. 1989 Nov;80(5):1128–1136. doi: 10.1161/01.cir.80.5.1128. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dunn M. J., Rose M. L., Latif N., Bradd S., Lovegrove C., Seymour C., Pomerance A., Yacoub M. H. Demonstration by western blotting of antiheart antibodies before and after cardiac transplantation. Transplantation. 1991 Apr;51(4):806–812. doi: 10.1097/00007890-199104000-00014. [DOI] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Paque R. E., Trousdale M. D., Gudvangen R. J., Barr D. T., Lipotich G. J., Nealon T. J., Duffey P. S. Temperature-sensitive mutant of coxsackievirus B3 establishes resistance in neonatal mice that protects them during adolescence against coxsackievirus B3-induced myocarditis. Infect Immun. 1983 Feb;39(2):851–864. doi: 10.1128/iai.39.2.851-864.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin O., Sole M. J., Butany J. W., Chia W. K., McLaughlin P. R., Liu P., Liew C. C. Detection of enterovirus RNA in myocardial biopsies from patients with myocarditis and cardiomyopathy using gene amplification by polymerase chain reaction. Circulation. 1990 Jul;82(1):8–16. doi: 10.1161/01.cir.82.1.8. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Why H., Richardson P., Archard L. Nucleotide sequencing of PCR products shows the presence of Coxsackie-B3 virus in endomyocardial biopsies from patients with myocarditis or dilated cardiomyopathy. Biochem Soc Trans. 1994 May;22(2):176S–176S. doi: 10.1042/bst022176s. [DOI] [PubMed] [Google Scholar]
- King M. L., Shaikh A., Bidwell D., Voller A., Banatvala J. E. Coxsackie-B-virus-specific IgM responses in children with insulin-dependent (juvenile-onset; type I) diabetes mellitus. Lancet. 1983 Jun 25;1(8339):1397–1399. doi: 10.1016/s0140-6736(83)92353-x. [DOI] [PubMed] [Google Scholar]
- Klingel K., Hohenadl C., Canu A., Albrecht M., Seemann M., Mall G., Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide H., Kitaura Y., Deguchi H., Ukimura A., Kawamura K., Hirai K. Genomic detection of enteroviruses in the myocardium--studies on animal hearts with coxsackievirus B3 myocarditis and endomyocardial biopsies from patients with myocarditis and dilated cardiomyopathy. Jpn Circ J. 1992 Oct;56(10):1081–1093. doi: 10.1253/jcj.56.1081. [DOI] [PubMed] [Google Scholar]
- Kutubuddin M., Simons J., Chow M. Identification of T-helper epitopes in the VP1 capsid protein of poliovirus. J Virol. 1992 May;66(5):3042–3047. doi: 10.1128/jvi.66.5.3042-3047.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kämmerer U., Kunkel B., Korn K. Nested PCR for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994 Feb;32(2):285–291. doi: 10.1128/jcm.32.2.285-291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif N., Baker C. S., Dunn M. J., Rose M. L., Brady P., Yacoub M. H. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS-PAGE and western blotting. J Am Coll Cardiol. 1993 Nov 1;22(5):1378–1384. doi: 10.1016/0735-1097(93)90546-d. [DOI] [PubMed] [Google Scholar]
- Leparc I., Fuchs F., Kopecka H., Aymard M. Use of the polymerase chain reaction with a murine model of picornavirus-induced myocarditis. J Clin Microbiol. 1993 Nov;31(11):2890–2894. doi: 10.1128/jcm.31.11.2890-2894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A., Wychowski C., Couderc T., Crainic R., Hogle J., Girard M. Engineering a poliovirus type 2 antigenic site on a type 1 capsid results in a chimaeric virus which is neurovirulent for mice. EMBO J. 1988 Sep;7(9):2839–2847. doi: 10.1002/j.1460-2075.1988.tb03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino T. A., Liu P., Sole M. J. Viral infection and the pathogenesis of dilated cardiomyopathy. Circ Res. 1994 Feb;74(2):182–188. doi: 10.1161/01.res.74.2.182. [DOI] [PubMed] [Google Scholar]
- Neumann D. A., Burek C. L., Baughman K. L., Rose N. R., Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990 Nov;16(6):839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- Nicholson F., Ajetunmobi J. F., Li M., Shackleton E. A., Starkey W. G., Illavia S. J., Muir P., Banatvala J. E. Molecular detection and serotypic analysis of enterovirus RNA in archival specimens from patients with acute myocarditis. Br Heart J. 1995 Nov;74(5):522–527. doi: 10.1136/hrt.74.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Zhang H., Bayston T. A., Archard L. C. Detection of Coxsackievirus B3 RNA in mouse myocarditis by nested polymerase chain reaction. Clin Diagn Virol. 1995 Mar;3(3):233–245. doi: 10.1016/s0928-0197(94)00040-9. [DOI] [PubMed] [Google Scholar]
- Prabhakar B. S., Srinivasappa J., Ray U. Selection of coxsackievirus B4 variants with monoclonal antibodies results in attenuation. J Gen Virol. 1987 Mar;68(Pt 3):865–869. doi: 10.1099/0022-1317-68-3-865. [DOI] [PubMed] [Google Scholar]
- Ramsingh A. I., Collins D. N. A point mutation in the VP4 coding sequence of coxsackievirus B4 influences virulence. J Virol. 1995 Nov;69(11):7278–7281. doi: 10.1128/jvi.69.11.7278-7281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann B. Y., Zell R., Kandolf R. Mapping of a neutralizing antigenic site of Coxsackievirus B4 by construction of an antigen chimera. J Virol. 1991 Jul;65(7):3475–3480. doi: 10.1128/jvi.65.7.3475-3480.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A., Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988 Apr;9(4):117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- Sabin A. B. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985 Mar;151(3):420–436. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- Schwaiger A., Umlauft F., Weyrer K., Larcher C., Lyons J., Mühlberger V., Dietze O., Grünewald K. Detection of enteroviral ribonucleic acid in myocardial biopsies from patients with idiopathic dilated cardiomyopathy by polymerase chain reaction. Am Heart J. 1993 Aug;126(2):406–410. doi: 10.1016/0002-8703(93)91058-m. [DOI] [PubMed] [Google Scholar]
- Severini G. M., Mestroni L., Falaschi A., Camerini F., Giacca M. Nested polymerase chain reaction for high-sensitivity detection of enteroviral RNA in biological samples. J Clin Microbiol. 1993 May;31(5):1345–1349. doi: 10.1128/jcm.31.5.1345-1349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Tu Z., Chapman N. M., Hufnagel G., Tracy S., Romero J. R., Barry W. H., Zhao L., Currey K., Shapiro B. The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5' nontranslated region. J Virol. 1995 Aug;69(8):4607–4618. doi: 10.1128/jvi.69.8.4607-4618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Billingham M. E., Cleary M. L. Detection of Coxsackievirus B3 RNA in myocardial tissues by the polymerase chain reaction. Am J Pathol. 1991 Feb;138(2):497–503. [PMC free article] [PubMed] [Google Scholar]
- Westrop G. D., Wareham K. A., Evans D. M., Dunn G., Minor P. D., Magrath D. I., Taffs F., Marsden S., Skinner M. A., Schild G. C. Genetic basis of attenuation of the Sabin type 3 oral poliovirus vaccine. J Virol. 1989 Mar;63(3):1338–1344. doi: 10.1128/jvi.63.3.1338-1344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Why H. J., Meany B. T., Richardson P. J., Olsen E. G., Bowles N. E., Cunningham L., Freeke C. A., Archard L. C. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation. 1994 Jun;89(6):2582–2589. doi: 10.1161/01.cir.89.6.2582. [DOI] [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., Austin M., Onodera T., Notkins A. L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979 May 24;300(21):1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- Yousef G. E., Bell E. J., Mann G. F., Murugesan V., Smith D. G., McCartney R. A., Mowbray J. F. Chronic enterovirus infection in patients with postviral fatigue syndrome. Lancet. 1988 Jan 23;1(8578):146–150. doi: 10.1016/s0140-6736(88)92722-5. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Cunningham L., Blake N. W., OuYang X., Bayston T. A., Kandolf R., Archard L. C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: The 5'-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993 Oct;41(2):129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]
- Zhang H., Blake N. W., Ouyang X., Pandolfino Y. A., Morgan-Capner P., Archard L. C. A single amino acid substitution in the capsid protein VP1 of coxsackievirus B3 (CVB3) alters plaque phenotype in Vero cells but not cardiovirulence in a mouse model. Arch Virol. 1995;140(5):959–966. doi: 10.1007/BF01314972. [DOI] [PubMed] [Google Scholar]
- Zhang H., Yousef G. E., Ouyang X., Archard L. C. Characterization of a murine model of myocarditis induced by a reactivated coxsackievirus B3. Int J Exp Pathol. 1994 Apr;75(2):99–110. [PMC free article] [PubMed] [Google Scholar]