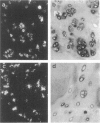
Abstract
Chondrocyte differentiation is characterized by distinct cellular phenotypes, which can be identified by specific extracellular matrix gene expression profiles. By applying in situ analysis on the mRNA and protein level in a series of benign and malignant human chondrogenic neoplasms, we were able to identify for the first time different phenotypes of neoplastic chondrocytes in vivo: 1) mature chondrocytes, which synthesized the characteristic cartilaginous extracellular tumor matrix, 2) cells resembling hypertrophic chondrocytes of the fetal growth plate, 3) cells resembling so-called dedifferentiated chondrocytes, and 4) well differentiated chondrocytic cells, which expressed type I collagen, indicating the presence of post-hypertrophic differentiated neoplastic chondrocytes. Chondrocytes exhibiting a range of phenotypes were found to be present in the same neoplasm. The different observed phenotypes, including the dedifferentiated phenotype, were in contrast to the anaplastic cells of high-grade chondrosarcomas. Comparison of expression data with tumor morphology revealed a relationship between the cellular phenotypes, the tumor matrix composition, and the matrix and cell morphology within the neoplasms. The distinctly different phenotypes of neoplastic chondrocytes are the basis of the characteristic high biochemical and morphological heterogeneity of chondroid neoplasms and shed light on their biological and clinical behavior.
Full text
PDF


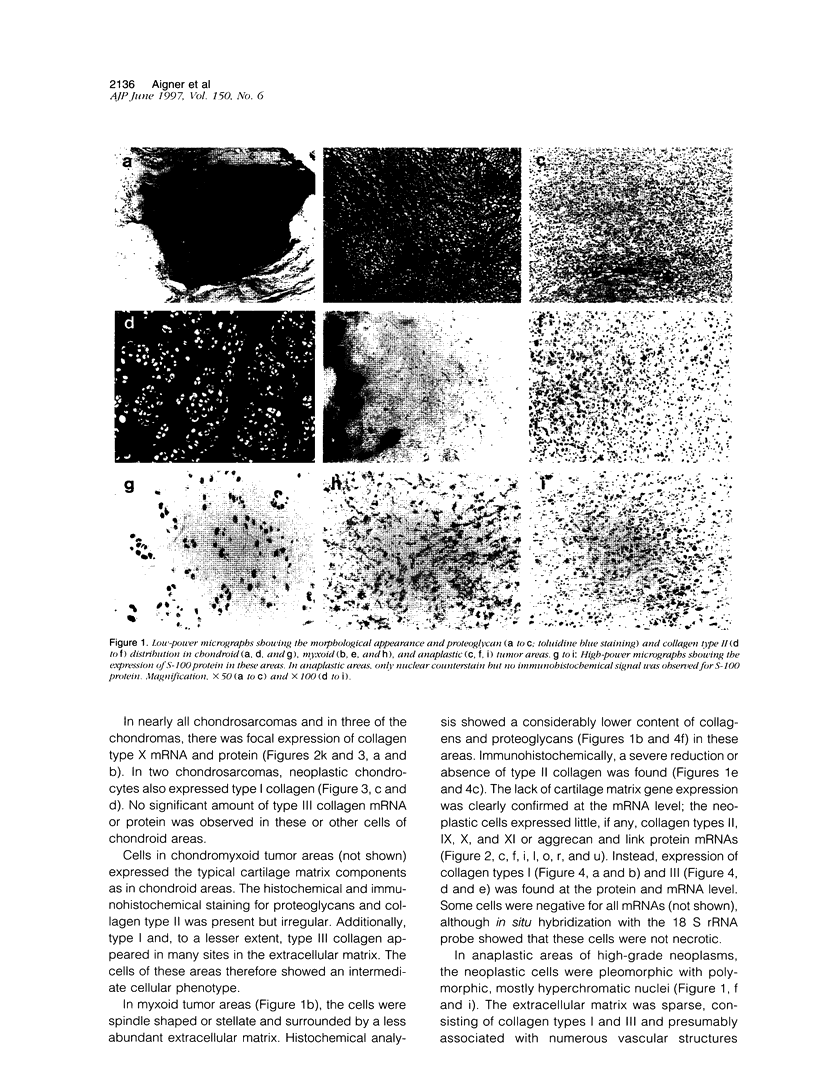
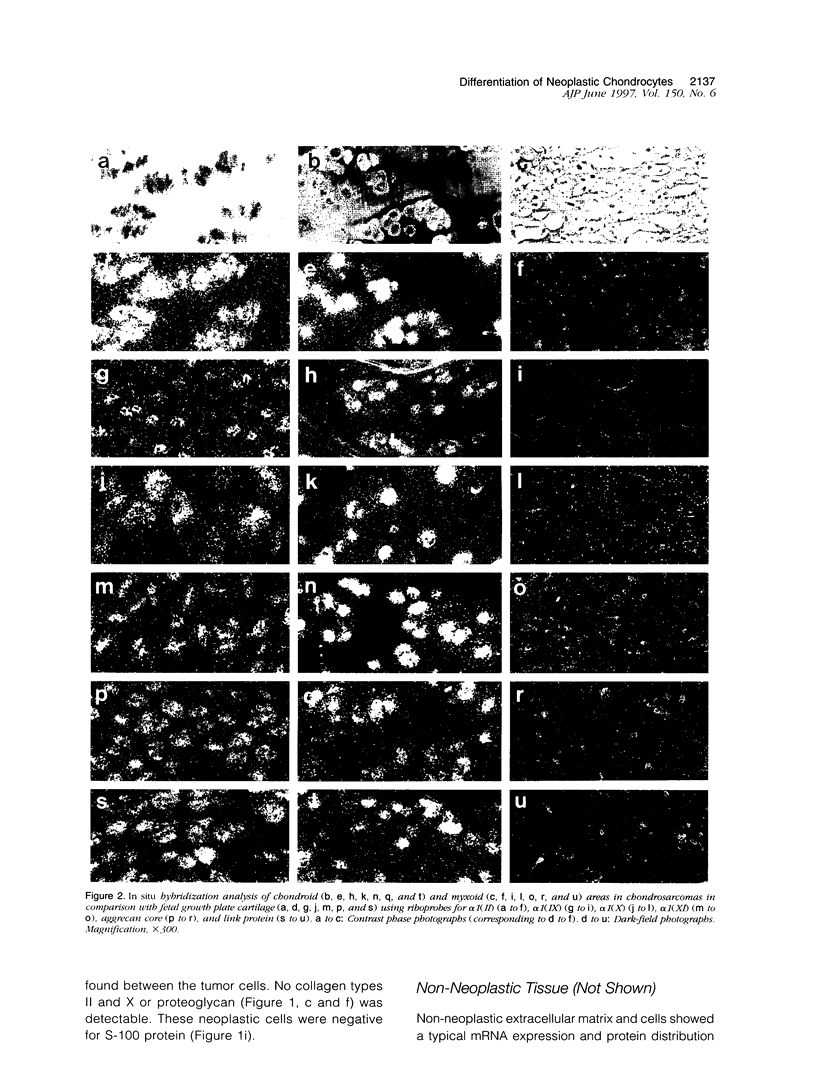
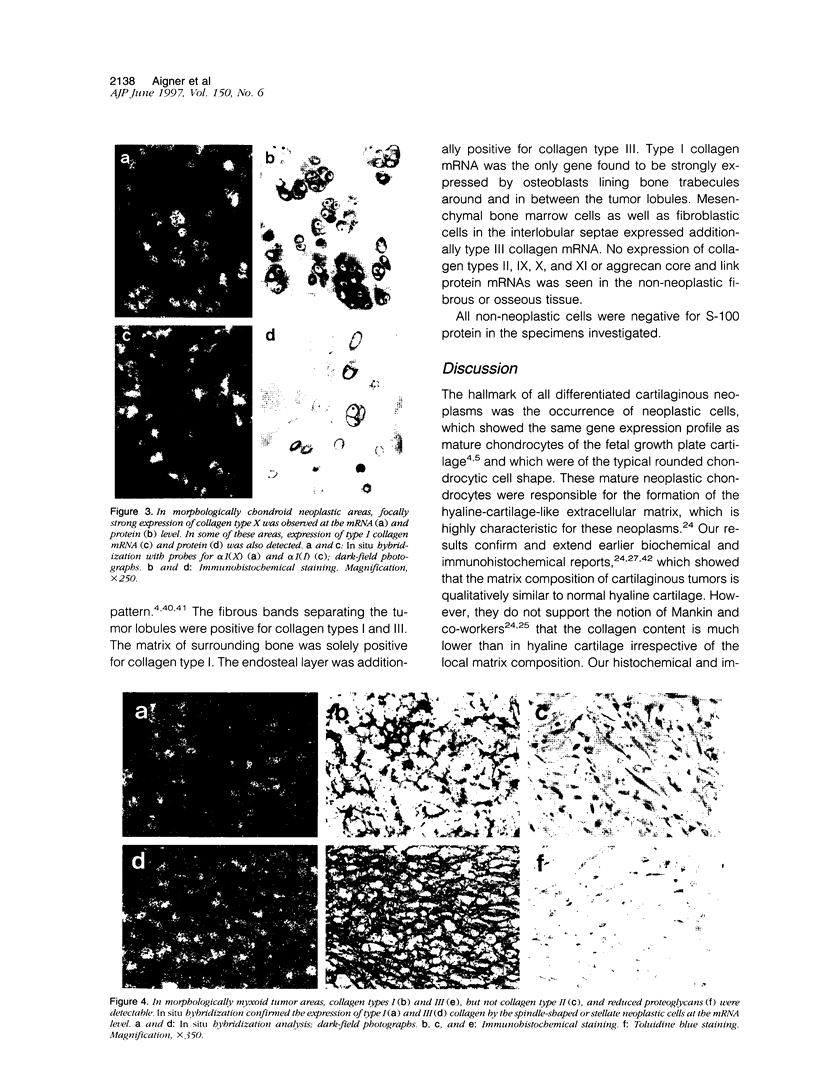



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T., Dietz U., Stöss H., von der Mark K. Differential expression of collagen types I, II, III, and X in human osteophytes. Lab Invest. 1995 Aug;73(2):236–243. [PubMed] [Google Scholar]
- Aigner T., Stöss H., Weseloh G., Zeiler G., von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(6):337–345. doi: 10.1007/BF02899701. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Brown P. D., Padilla S. R. Microfilament modification by dihydrocytochalasin B causes retinoic acid-modulated chondrocytes to reexpress the differentiated collagen phenotype without a change in shape. J Cell Biol. 1988 Jan;106(1):161–170. doi: 10.1083/jcb.106.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. The progeny of rabbit articular chondrocytes synthesize collagen types I and III and type I trimer, but not type II. Verifications by cyanogen bromide peptide analysis. Biochemistry. 1977 Mar 8;16(5):865–872. doi: 10.1021/bi00624a009. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonaventure J., Kadhom N., Cohen-Solal L., Ng K. H., Bourguignon J., Lasselin C., Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994 May;212(1):97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- Brewton R. G., Wright D. W., Mayne R. Structural and functional comparison of type IX collagen-proteoglycan from chicken cartilage and vitreous humor. J Biol Chem. 1991 Mar 15;266(8):4752–4757. [PubMed] [Google Scholar]
- Cancedda R., Descalzi Cancedda F., Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. doi: 10.1016/s0074-7696(08)62109-9. [DOI] [PubMed] [Google Scholar]
- Caplan A. I. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Carter D. H., Sloan P., Aaron J. E. Immunolocalization of collagen types I and III, tenascin, and fibronectin in intramembranous bone. J Histochem Cytochem. 1991 May;39(5):599–606. doi: 10.1177/39.5.1707904. [DOI] [PubMed] [Google Scholar]
- Descalzi Cancedda F., Gentili C., Manduca P., Cancedda R. Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol. 1992 Apr;117(2):427–435. doi: 10.1083/jcb.117.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhia J., Davidson C. M., Wells T. M., Vynios D. H., Hardingham T. E., Bayliss M. T. Age-related changes in the content of the C-terminal region of aggrecan in human articular cartilage. Biochem J. 1996 Feb 1;313(Pt 3):933–940. doi: 10.1042/bj3130933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhia J., Hardingham T. E. The primary structure of human cartilage link protein. Nucleic Acids Res. 1990 Mar 11;18(5):1292–1292. doi: 10.1093/nar/18.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. L., Ayala A. G., Romsdahl M. M. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977 Aug;40(2):818–831. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Finer M. H., Gerstenfeld L. C., Young D., Doty P., Boedtker H. Collagen expression in embryonic chicken chondrocytes treated with phorbol myristate acetate. Mol Cell Biol. 1985 Jun;5(6):1415–1424. doi: 10.1128/mcb.5.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. S., Kay S. A comparative ultrastructural study of mesenchymal chondrosarcoma and myxoid chondrosarcoma. Cancer. 1974 Jun;33(6):1531–1542. doi: 10.1002/1097-0142(197406)33:6<1531::aid-cncr2820330610>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Girkontaite I., Frischholz S., Lammi P., Wagner K., Swoboda B., Aigner T., Von der Mark K. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996 Sep;15(4):231–238. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J. R., Suen L. F., Yamin R., Mizuno S., Glowacki J., Arbiser J. L., Apperley J. F. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994 Dec;94(6):2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E. T., Jr, Luyten F. P., Reddi A. H. Osteogenin promotes reexpression of cartilage phenotype by dedifferentiated articular chondrocytes in serum-free medium. Exp Cell Res. 1991 Feb;192(2):340–345. doi: 10.1016/0014-4827(91)90050-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Seki K., Yang P., Hirose T., Hizawa K., Wada T., Wakabayashi J. Differentiation and proliferative activity in benign and malignant cartilage tumors of bone. Hum Pathol. 1995 Aug;26(8):838–845. doi: 10.1016/0046-8177(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Hiltunen A., Aro H. T., Vuorio E. Regulation of extracellular matrix genes during fracture healing in mice. Clin Orthop Relat Res. 1993 Dec;(297):23–27. [PubMed] [Google Scholar]
- Holtzer H., Abbott J., Lash J., Holtzer S. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima A., Ueda Y., Tsuchiya H., Tomita K., Nagai Y., Nakanishi I. Immunohistochemical localization of collagenous proteins in cartilaginous tumors: characteristic distribution of type IX collagen. J Cancer Res Clin Oncol. 1993;120(1-2):35–40. doi: 10.1007/BF01200722. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Swoboda B., von der Mark K. Ascorbate independent differentiation of human chondrocytes in vitro: simultaneous expression of types I and X collagen and matrix mineralization. Differentiation. 1992 Dec;52(1):89–100. doi: 10.1111/j.1432-0436.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Johnell O., Hulth A., Holmdahl R., Rubin K. Reactivity of monoclonal anti-type II collagen antibodies with cartilage and synovial tissue in rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1986 Jun;29(6):730–738. doi: 10.1002/art.1780290605. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Cantley K. P., Lippiello L., Schiller A. L., Campbell C. J. The biology of human chondrosarcoma. I. Description of the cases, grading, and biochemical analyses. J Bone Joint Surg Am. 1980 Mar;62(2):160–176. [PubMed] [Google Scholar]
- Mankin H. J., Cantley K. P., Schiller A. L., Lippiello L. The biology of human chondrosarcoma. II. Variation in chemical composition among types and subtypes of benign and malignant cartilage tumors. J Bone Joint Surg Am. 1980 Mar;62(2):176–188. [PubMed] [Google Scholar]
- Mayne R., Brewton R. G., Mayne P. M., Baker J. R. Isolation and characterization of the chains of type V/type XI collagen present in bovine vitreous. J Biol Chem. 1993 May 5;268(13):9381–9386. [PubMed] [Google Scholar]
- Mundlos S., Meyer R., Yamada Y., Zabel B. Distribution of cartilage proteoglycan (aggrecan) core protein and link protein gene expression during human skeletal development. Matrix. 1991 Nov;11(5):339–346. doi: 10.1016/s0934-8832(11)80205-2. [DOI] [PubMed] [Google Scholar]
- Müller P. K., Lemmen C., Gay S., Gauss V., Kühn K. Immunochemical and biochemical study of collagen synthesis by chondrocytes in culture. Exp Cell Res. 1977 Aug;108(1):47–55. [PubMed] [Google Scholar]
- Okajima K., Honda I., Kitagawa T. Immunohistochemical distribution of S-100 protein in tumors and tumor-like lesions of bone and cartilage. Cancer. 1988 Feb 15;61(4):792–799. doi: 10.1002/1097-0142(19880215)61:4<792::aid-cncr2820610425>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Povýsil C., Matejovský Z. A comparative ultrastructural study of chondrosarcoma, chordoid sarcoma, chordoma and chordoma periphericum. Pathol Res Pract. 1985 Mar;179(4-5):546–559. doi: 10.1016/S0344-0338(85)80196-5. [DOI] [PubMed] [Google Scholar]
- Quarto R., Dozin B., Bonaldo P., Cancedda R., Colombatti A. Type VI collagen expression is upregulated in the early events of chondrocyte differentiation. Development. 1993 Jan;117(1):245–251. doi: 10.1242/dev.117.1.245. [DOI] [PubMed] [Google Scholar]
- Reichenberger E., Aigner T., von der Mark K., Stöss H., Bertling W. In situ hybridization studies on the expression of type X collagen in fetal human cartilage. Dev Biol. 1991 Dec;148(2):562–572. doi: 10.1016/0012-1606(91)90274-7. [DOI] [PubMed] [Google Scholar]
- Remberger K., Gay S. Immunhistochemical demonstration of different collagen types in the normal epiphyseal plate and in benign and malignant tumors of bone and cartilage. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1977 Oct;90(1):95–106. doi: 10.1007/BF00306024. [DOI] [PubMed] [Google Scholar]
- Roach H. I., Erenpreisa J., Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995 Oct;131(2):483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronot X., Hecquet C., Jaffray P., Guiguet M., Adolphe M., Fontagne J., Lechat P. Proliferation kinetics of rabbit articular chondrocytes in primary culture and at the first passage. Cell Tissue Kinet. 1983 Nov;16(6):531–537. [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Sandberg M., Autio-Harmainen H., Vuorio E. Localization of the expression of types I, III, and IV collagen, TGF-beta 1 and c-fos genes in developing human calvarial bones. Dev Biol. 1988 Nov;130(1):324–334. doi: 10.1016/0012-1606(88)90438-1. [DOI] [PubMed] [Google Scholar]
- Sandberg M., Vuorio E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J Cell Biol. 1987 Apr;104(4):1077–1084. doi: 10.1083/jcb.104.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. J., Morris N., Robbins J. R., Goldring M. B. Alternatively spliced type II procollagen mRNAs define distinct populations of cells during vertebral development: differential expression of the amino-propeptide. J Cell Biol. 1991 Sep;114(6):1307–1319. doi: 10.1083/jcb.114.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T. M., Linsenmayer T. F. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985 Feb;100(2):598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y., Oda Y., Tsuchiya H., Tomita K., Nakanishi I. Immunohistological study on collagenous proteins of benign and malignant human cartilaginous tumours of bone. Virchows Arch A Pathol Anat Histopathol. 1990;417(4):291–297. doi: 10.1007/BF01605779. [DOI] [PubMed] [Google Scholar]
- Vornehm S. I., Dudhia J., Von der Mark K., Aigner T. Expression of collagen types IX and XI and other major cartilage matrix components by human fetal chondrocytes in vivo. Matrix Biol. 1996 Jul;15(2):91–98. doi: 10.1016/s0945-053x(96)90150-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jimenez S. A., Kaji A. Effects of viral transformation on synthesis and secretion of collagen and fibronectin-like molecules by embryonic chick chondrocytes in culture. J Biol Chem. 1981 Sep 10;256(17):9111–9117. [PubMed] [Google Scholar]
- von der Mark K., Gauss V., von der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]