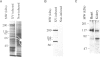Abstract
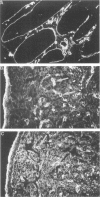
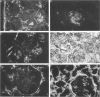
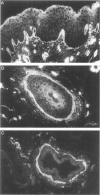
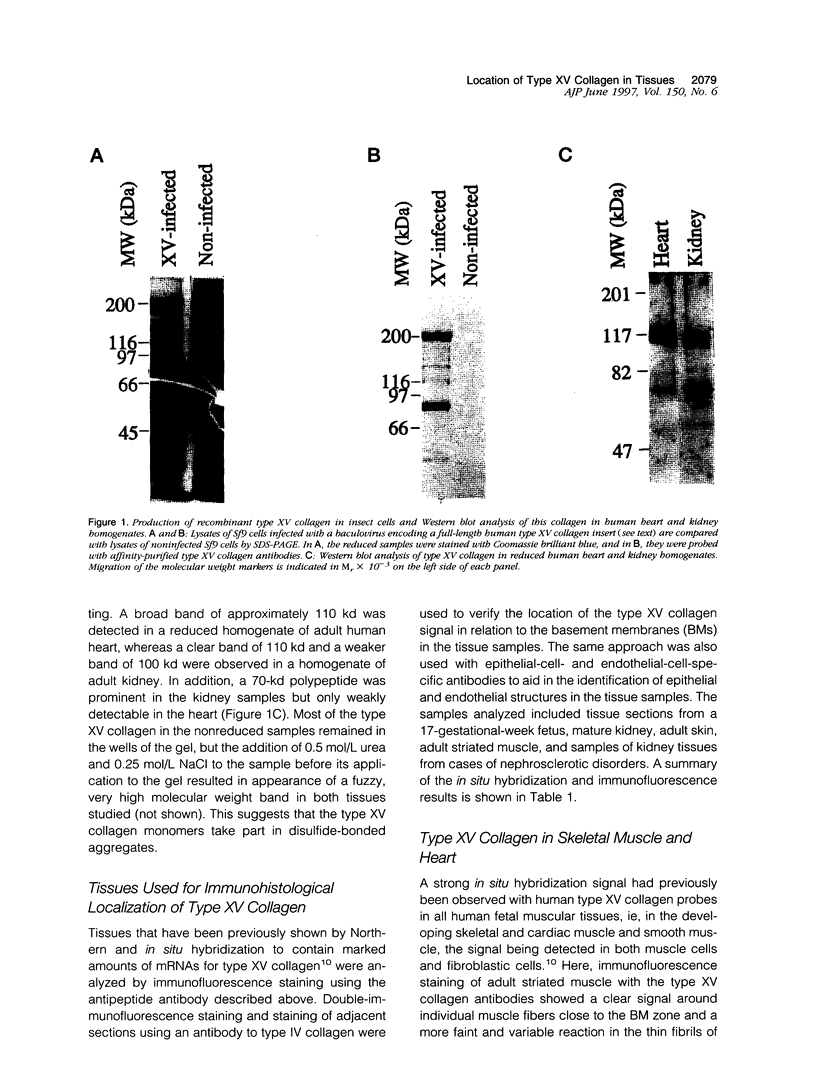
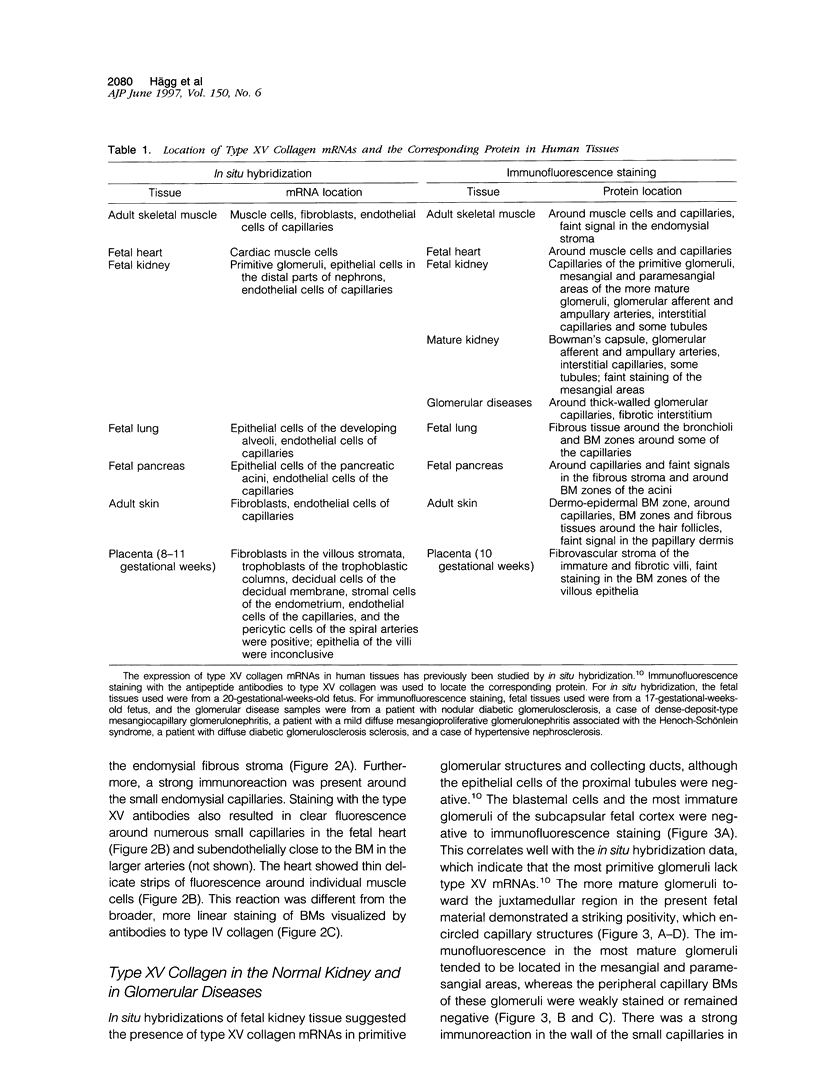
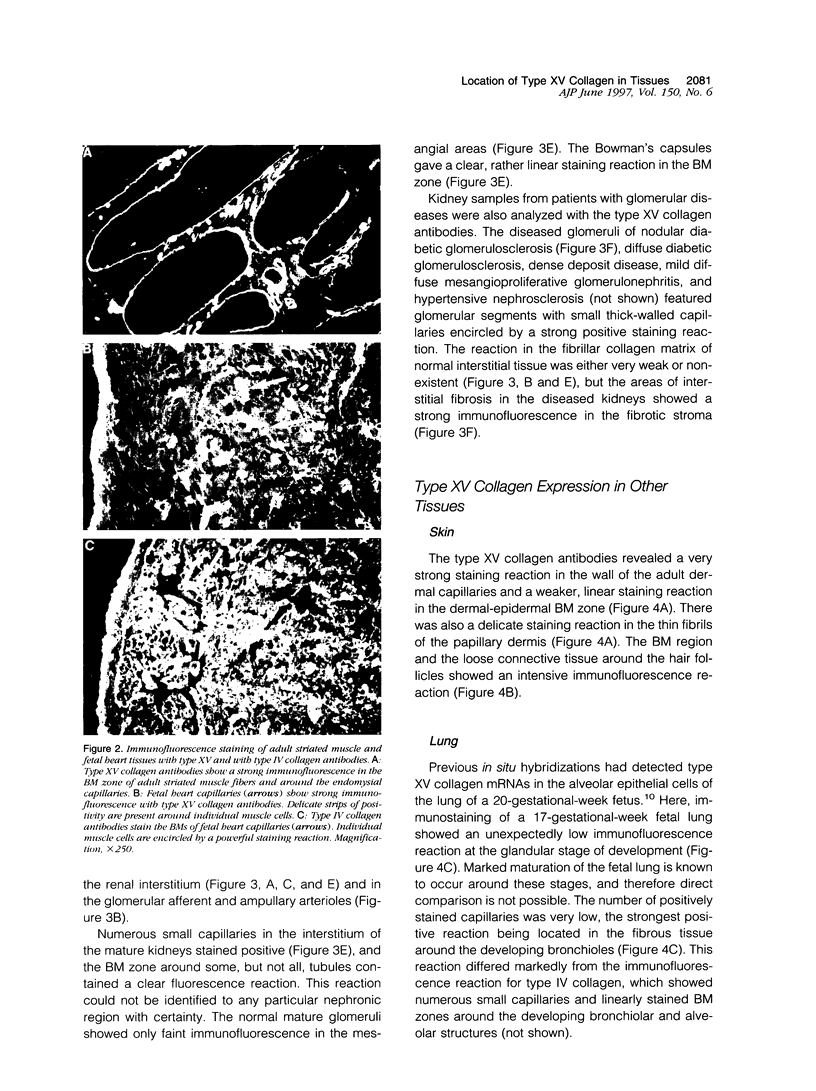
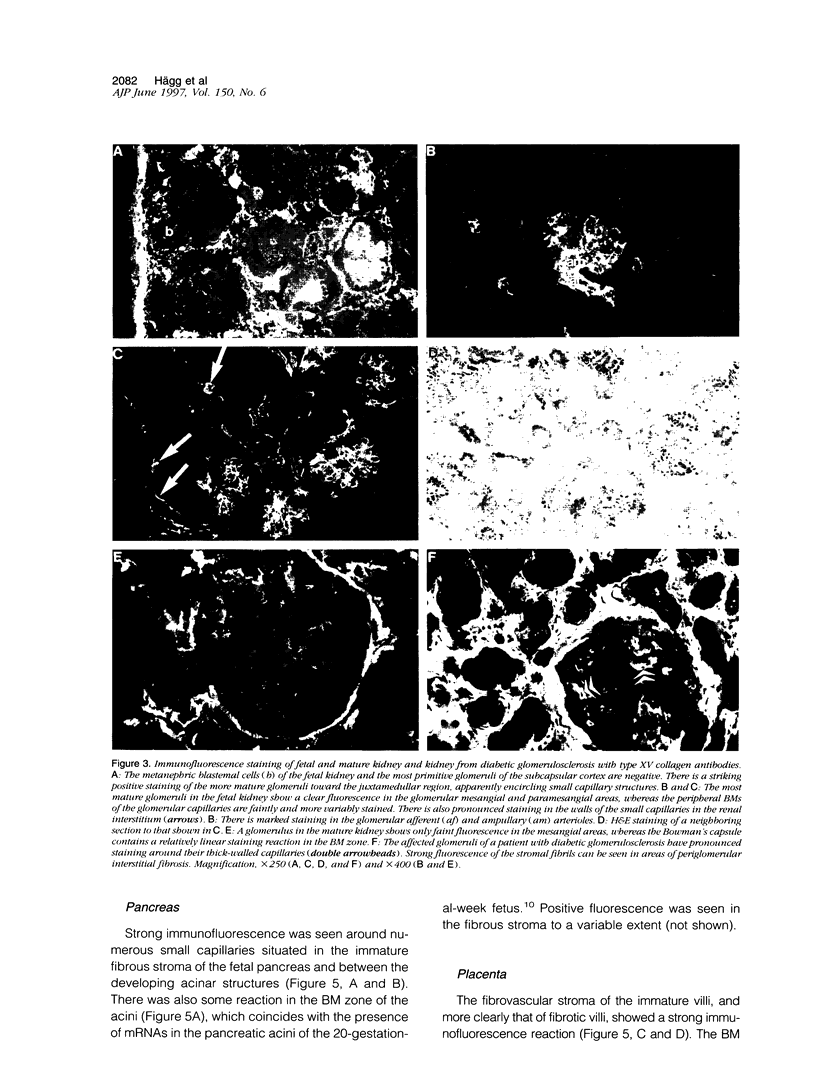
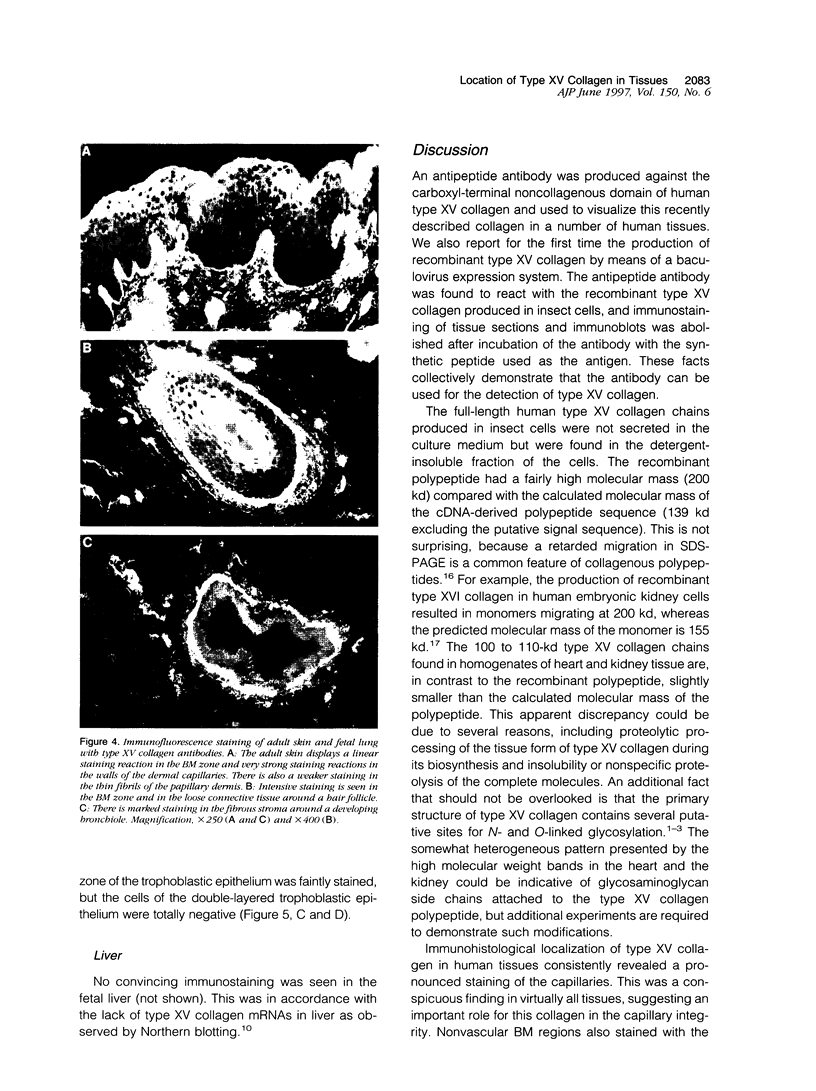
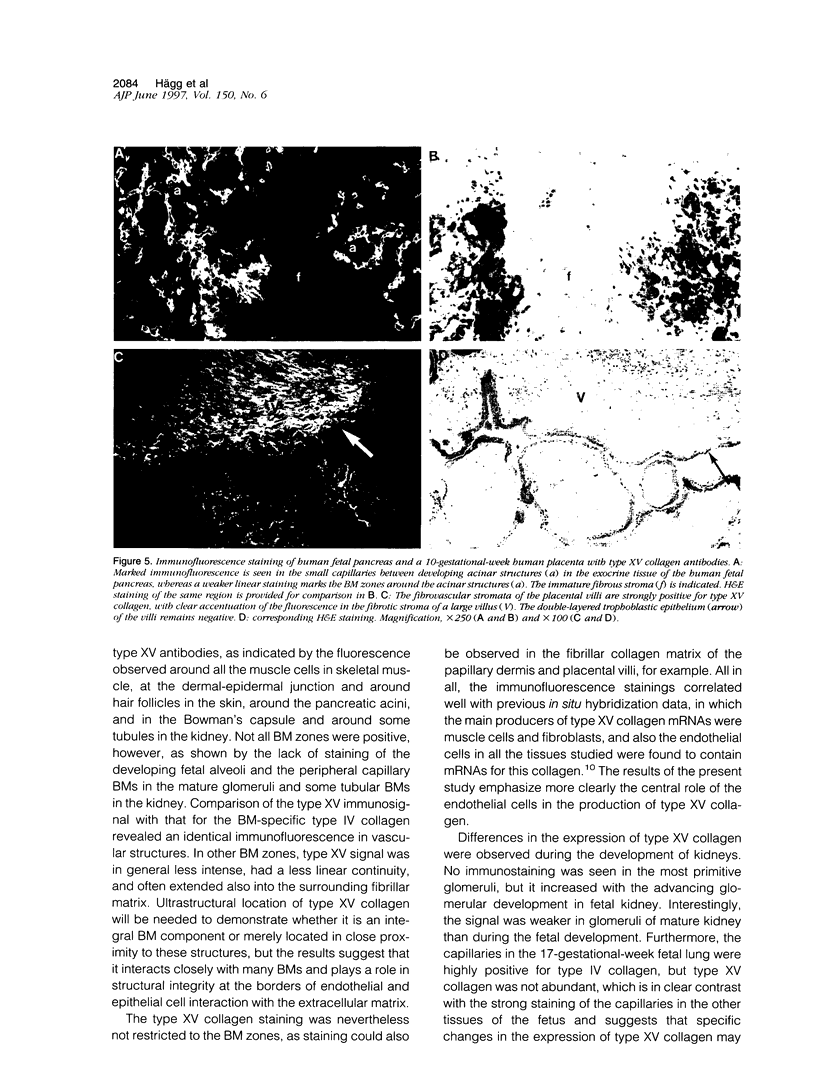
An antipeptide antibody was produced against the carboxyl-terminal noncollagenous domain of human type XV collagen and used to localize this recently described collagen in a number of human tissues. The most conspicuous findings were powerful staining of most of the capillaries and staining of the basement membrane (BM) zones of muscle cells. Not all of the BM zones were positive, however, as shown by the lack of staining in the developing fetal alveoli and some of the tubules in developing kidney. Nor was type XV collagen staining restricted to the BM zones, as some could be observed in the fibrillar collagen matrix of the papillary dermis and placental villi, for example. Interestingly, differences in the expression of type XV collagen could be observed during kidney development, and staining of fetal lung tissue suggested that changes in its expression may also occur during the formation of vascular structures. Another intriguing finding was pronounced renal interstitial type XV collagen staining in patients with kidney fibrosis due to different pathological processes. This suggests that the accumulation of type XV collagen may accompany fibrotic processes. Full-length human type XV collagen chains with an apparent molecular mass of approximately 200 kd were produced in insect cells using a baculovirus expression system. The fact that these had a markedly higher molecular mass than the 100- to 110-kd type XV collagen chains found in homogenates of heart and kidney tissue suggests either proteolytic processing during the synthesis of type XV collagen or an inability to solubilize complete molecules from tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisswenger P. J., Spiro R. G. Studies on the human glomerular basement membrane. Composition, nature of the carbohydrate units and chemical changes in diabetes mellitus. Diabetes. 1973 Mar;22(3):180–193. doi: 10.2337/diab.22.3.180. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Scheinman J. I., Mauer S. M., Michael A. F. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983 May;32 (Suppl 2):34–39. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- Kim Y., Kleppel M. M., Butkowski R., Mauer S. M., Wieslander J., Michael A. F. Differential expression of basement membrane collagen chains in diabetic nephropathy. Am J Pathol. 1991 Feb;138(2):413–420. [PMC free article] [PubMed] [Google Scholar]
- Kivirikko S., Heinämäki P., Rehn M., Honkanen N., Myers J. C., Pihlajaniemi T. Primary structure of the alpha 1 chain of human type XV collagen and exon-intron organization in the 3' region of the corresponding gene. J Biol Chem. 1994 Feb 18;269(7):4773–4779. [PubMed] [Google Scholar]
- Kivirikko S., Saarela J., Myers J. C., Autio-Harmainen H., Pihlajaniemi T. Distribution of type XV collagen transcripts in human tissue and their production by muscle cells and fibroblasts. Am J Pathol. 1995 Nov;147(5):1500–1509. [PMC free article] [PubMed] [Google Scholar]
- Kolbe M., Kaufman J. L., Friedman J., Dinerstein C., Mackenzie J. W., Boyd C. D. Changes in steady-state levels of mRNAs coding for type IV collagen, laminin and fibronectin following capillary basement membrane thickening in human adult onset diabetes. Connect Tissue Res. 1990;25(1):77–85. doi: 10.3109/03008209009009814. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamberg A., Helaakoski T., Myllyharju J., Peltonen S., Notbohm H., Pihlajaniemi T., Kivirikko K. I. Characterization of human type III collagen expressed in a baculovirus system. Production of a protein with a stable triple helix requires coexpression with the two types of recombinant prolyl 4-hydroxylase subunit. J Biol Chem. 1996 May 17;271(20):11988–11995. doi: 10.1074/jbc.271.20.11988. [DOI] [PubMed] [Google Scholar]
- Malitschek B., Schartl M. Rapid identification of recombinant baculoviruses using PCR. Biotechniques. 1991 Aug;11(2):177–178. [PubMed] [Google Scholar]
- Miller E. J., Rhodes R. K. Preparation and characterization of the different types of collagen. Methods Enzymol. 1982;82(Pt A):33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- Muragaki Y., Abe N., Ninomiya Y., Olsen B. R., Ooshima A. The human alpha 1(XV) collagen chain contains a large amino-terminal non-triple helical domain with a tandem repeat structure and homology to alpha 1(XVIII) collagen. J Biol Chem. 1994 Feb 11;269(6):4042–4046. [PubMed] [Google Scholar]
- Muragaki Y., Timmons S., Griffith C. M., Oh S. P., Fadel B., Quertermous T., Olsen B. R. Mouse Col18a1 is expressed in a tissue-specific manner as three alternative variants and is localized in basement membrane zones. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8763–8767. doi: 10.1073/pnas.92.19.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Kivirikko S., Gordon M. K., Dion A. S., Pihlajaniemi T. Identification of a previously unknown human collagen chain, alpha 1(XV), characterized by extensive interruptions in the triple-helical region. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10144–10148. doi: 10.1073/pnas.89.21.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. P., Kamagata Y., Muragaki Y., Timmons S., Ooshima A., Olsen B. R. Isolation and sequencing of cDNAs for proteins with multiple domains of Gly-Xaa-Yaa repeats identify a distinct family of collagenous proteins. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4229–4233. doi: 10.1073/pnas.91.10.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S. P., Warman M. L., Seldin M. F., Cheng S. D., Knoll J. H., Timmons S., Olsen B. R. Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the alpha 1(XVIII) collagen gene to mouse chromosome 10 and human chromosome 21. Genomics. 1994 Feb;19(3):494–499. doi: 10.1006/geno.1994.1098. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Myllylä R., Kivirikko K. I., Tryggvason K. Effects of streptozotocin diabetes, glucose, and insulin on the metabolism of type IV collagen and proteoglycan in murine basement membrane-forming EHS tumor tissue. J Biol Chem. 1982 Dec 25;257(24):14914–14920. [PubMed] [Google Scholar]
- Pihlajaniemi T., Rehn M. Two new collagen subgroups: membrane-associated collagens and types XV and XVII. Prog Nucleic Acid Res Mol Biol. 1995;50:225–262. doi: 10.1016/s0079-6603(08)60816-8. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Rehn M., Hintikka E., Pihlajaniemi T. Primary structure of the alpha 1 chain of mouse type XVIII collagen, partial structure of the corresponding gene, and comparison of the alpha 1(XVIII) chain with its homologue, the alpha 1(XV) collagen chain. J Biol Chem. 1994 May 13;269(19):13929–13935. [PubMed] [Google Scholar]
- Rehn M., Pihlajaniemi T. Alpha 1(XVIII), a collagen chain with frequent interruptions in the collagenous sequence, a distinct tissue distribution, and homology with type XV collagen. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4234–4238. doi: 10.1073/pnas.91.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillet E., Mann K., Nischt R., Pan T. C., Chu M. L., Timpl R. Recombinant analysis of human alpha 1 (XVI) collagen. Evidence for processing of the N-terminal globular domain. Eur J Biochem. 1995 Feb 15;228(1):160–168. doi: 10.1111/j.1432-1033.1995.tb20245.x. [DOI] [PubMed] [Google Scholar]
- van den Born J., van Kraats A. A., Bakker M. A., Assmann K. J., van den Heuvel L. P., Veerkamp J. H., Berden J. H. Selective proteinuria in diabetic nephropathy in the rat is associated with a relative decrease in glomerular basement membrane heparan sulphate. Diabetologia. 1995 Feb;38(2):161–172. doi: 10.1007/BF00400090. [DOI] [PubMed] [Google Scholar]