Abstract
P-glycoprotein (P-gp) expels hydrophobic substances from the cell, including chemotherapeutic agents and immunosuppressants such as cyclosporin A (CsA) and FK506. Exposure of cultured renal tubular cells to CsA induces P-gp overexpression in cell membranes. Angiotensin II has recently been implicated as the principal factor responsible for progression of interstitial fibrosis induced by CsA. To investigate the in vivo relationships between histological lesions, P-gp overexpression, and intrarenal angiotensin II deposits, we developed a model of chronic CsA toxicity in Sprague-Dawley rats treated with 25 mg/kg/day CsA for 28 and 56 days and fed either a standard maintenance diet or a low-salt diet. Immunohistochemical methods were used to study the expression of P-gp in renal tubular cells and the appearance of intrarenal angiotensin II deposits. Rats treated with CsA developed chronic nephrotoxicity lesions that were more evident in the group fed the low-salt diet. Treatment with CsA induced overexpression of P-gp in tubular cells of the kidney that increased with time. We found that immunohistochemical expression of P-gp was slightly more severe in rats fed a low-salt diet. Intrarenal deposits of angiotensin II were more evident in rats treated with CsA; these deposits also increased with time. This finding was also more relevant in rats given the low-salt diet. The up-regulation of P-gp was inversely related to the incidence of hyaline arteriopathy (r = -0.65; P < 0.05), periglomerular (r = -0.58; P < 0.05) and peritubular fibrosis (r = -0.63; P < 0.05), and intrarenal angiotensin H deposits in animals with severe signs of nephrotoxicity (r = -0.65; P < 0.05). These results support the hypothesis that the role of P-gp as a detoxicant in renal cells may be related to mechanisms that control the cytoplasmic removal of both toxic metabolites from CsA and those originating from the catabolism of signal transduction proteins (methylcysteine esters), which are produced as a result of ras activation in presence of angiotensin II.
Full text
PDF


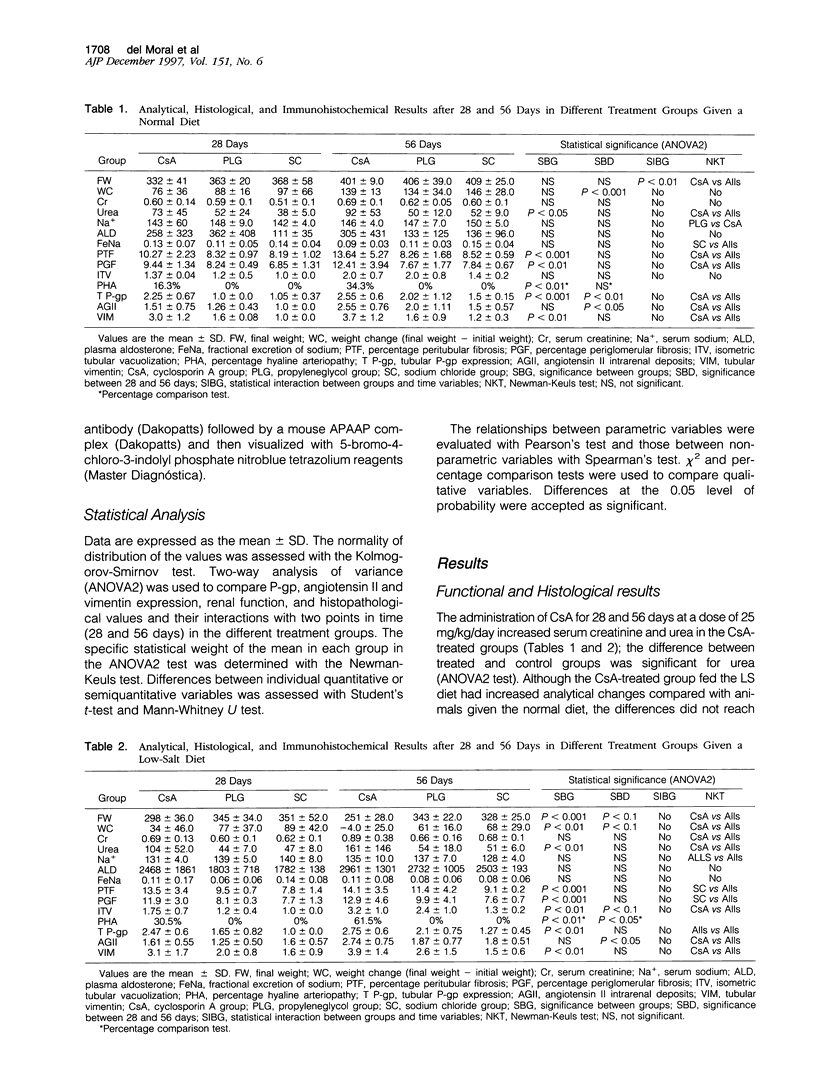

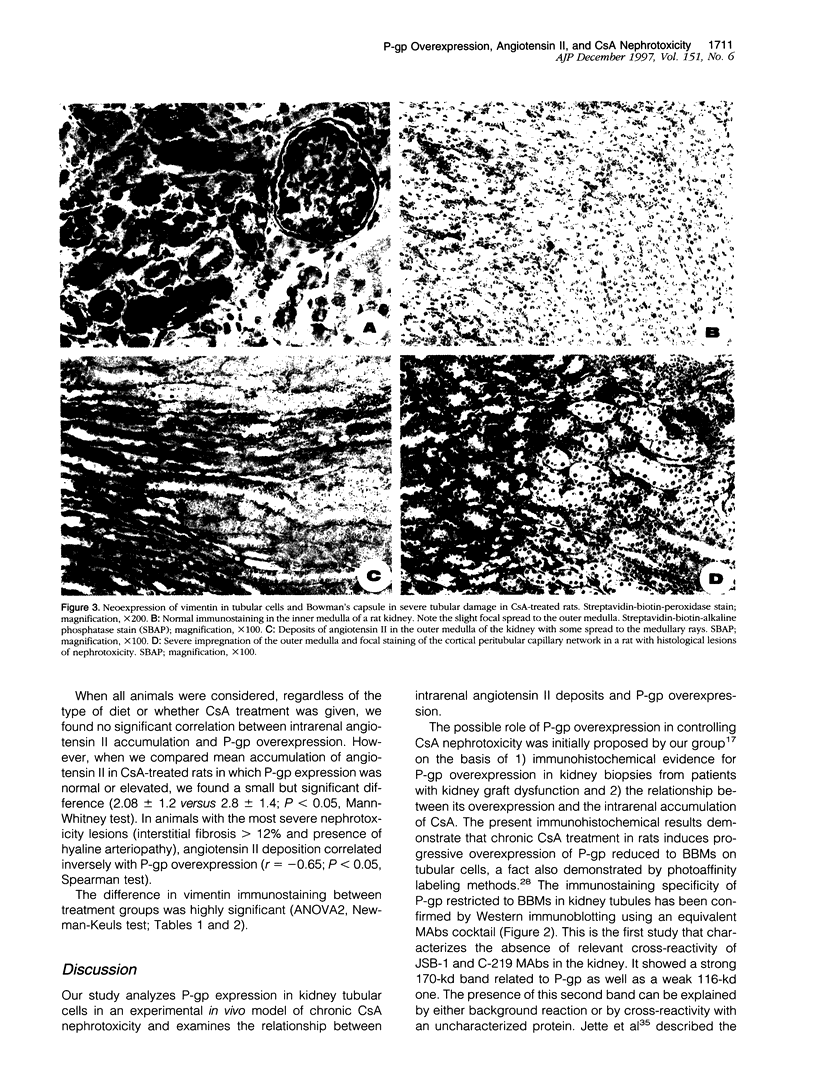



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai H., Escobedo J. A. Angiotensin II type 1 receptor signals through Raf-1 by a protein kinase C-dependent, Ras-independent mechanism. Mol Pharmacol. 1996 Sep;50(3):522–528. [PubMed] [Google Scholar]
- Bennett W. M., DeMattos A., Meyer M. M., Andoh T., Barry J. M. Chronic cyclosporine nephropathy: the Achilles' heel of immunosuppressive therapy. Kidney Int. 1996 Oct;50(4):1089–1100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- Border W. A., Noble N. A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994 Nov 10;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Burdmann E. A., Andoh T. F., Nast C. C., Evan A., Connors B. A., Coffman T. M., Lindsley J., Bennett W. M. Prevention of experimental cyclosporin-induced interstitial fibrosis by losartan and enalapril. Am J Physiol. 1995 Oct;269(4 Pt 2):F491–F499. doi: 10.1152/ajprenal.1995.269.4.F491. [DOI] [PubMed] [Google Scholar]
- Buss W. C., Stepanek J., Queen S. A. Association of tissue-specific changes in translation elongation after cyclosporin with changes in elongation factor 2 phosphorylation. Biochem Pharmacol. 1994 Oct 7;48(7):1459–1469. doi: 10.1016/0006-2952(94)90571-1. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., O'Brien J. P., Boccia J., Casals D., Bertino J. R., Melamed M. R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990 Sep;38(9):1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Devarajan P., Kaskel F. J., Arbeit L. A., Moore L. C. Cyclosporine nephrotoxicity: blood volume, sodium conservation, and renal hemodynamics. Am J Physiol. 1989 Jan;256(1 Pt 2):F71–F78. doi: 10.1152/ajprenal.1989.256.1.F71. [DOI] [PubMed] [Google Scholar]
- Diamond J. R., Kees-Folts D., Ding G., Frye J. E., Restrepo N. C. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994 Jun;266(6 Pt 2):F926–F933. doi: 10.1152/ajprenal.1994.266.6.F926. [DOI] [PubMed] [Google Scholar]
- Elzinga L. W., Rosen S., Bennett W. M. Dissociation of glomerular filtration rate from tubulointerstitial fibrosis in experimental chronic cyclosporine nephropathy: role of sodium intake. J Am Soc Nephrol. 1993 Aug;4(2):214–221. doi: 10.1681/ASN.V42214. [DOI] [PubMed] [Google Scholar]
- Ember I., Kiss I., Dezsényi E., Kertai P. Early effects of cyclosporin A on in vivo oncogene expression. Anticancer Res. 1994 May-Jun;14(3A):1095–1096. [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant T. W., Silverman J. A., Thorgeirsson S. S. Regulation of P-glycoprotein gene expression in hepatocyte cultures and liver cell lines by a trans-acting transcriptional repressor. Nucleic Acids Res. 1992 Jun 11;20(11):2841–2846. doi: 10.1093/nar/20.11.2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García del Moral R., O'Valle F., Andújar M., Aguilar M., Lucena M. A., López-Hidalgo J., Ramírez C., Medina-Cano M. T., Aguilar D., Gómez-Morales M. Relationship between P-glycoprotein expression and cyclosporin A in kidney. An immunohistological and cell culture study. Am J Pathol. 1995 Feb;146(2):398–408. [PMC free article] [PubMed] [Google Scholar]
- Gerkens J. F., Bhagwandeen S. B., Dosen P. J., Smith A. J. The effect of salt intake on cyclosporine-induced impairment of renal function in rats. Transplantation. 1984 Oct;38(4):412–417. doi: 10.1097/00007890-198410000-00019. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hyde S. C., Higgins C. F., Valverde M. A., Mintenig G. M., Sepúlveda F. V. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992 Oct 2;71(1):23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. How cancer cells evade chemotherapy: sixteenth Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1993 Feb 15;53(4):747–754. [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gómez-Morales M., Bustos M., Montes A., Andújar M., Medina-Cano M. T., Ramírez C., O'Valle F., Aguilar D., Aneiros J., García del Moral R. Influence of intrarenal deposits of ciclosporin A on acute renal transplant rejection. Nephron. 1995;70(4):402–409. doi: 10.1159/000188636. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature. 1993 Sep 23;365(6444):352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- Jetté L., Beaulieu E., Leclerc J. M., Béliveau R. Cyclosporin A treatment induces overexpression of P-glycoprotein in the kidney and other tissues. Am J Physiol. 1996 May;270(5 Pt 2):F756–F765. doi: 10.1152/ajprenal.1996.270.5.F756. [DOI] [PubMed] [Google Scholar]
- Jetté L., Pouliot J. F., Murphy G. F., Béliveau R. Isoform I (mdr3) is the major form of P-glycoprotein expressed in mouse brain capillaries. Evidence for cross-reactivity of antibody C219 with an unrelated protein. Biochem J. 1995 Feb 1;305(Pt 3):761–766. doi: 10.1042/bj3050761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Alpers C. E., Yoshimura A., Lombardi D., Pritzl P., Floege J., Schwartz S. M. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992 May;19(5):464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Khanna A., Li B., Stenzel K. H., Suthanthiran M. Regulation of new DNA synthesis in mammalian cells by cyclosporine. Demonstration of a transforming growth factor beta-dependent mechanism of inhibition of cell growth. Transplantation. 1994 Feb 27;57(4):577–582. [PubMed] [Google Scholar]
- Kim W. J., Kakehi Y., Kinoshita H., Arao S., Fukumoto M., Yoshida O. Expression patterns of multidrug-resistance (MDR1), multidrug resistance-associated protein (MRP),glutathione-S-transferase-pi (GST-pi) and DNA topoisomerase II (Topo II) genes in renal cell carcinomas and normal kidney. J Urol. 1996 Aug;156(2 Pt 1):506–511. doi: 10.1097/00005392-199608000-00072. [DOI] [PubMed] [Google Scholar]
- Kon V., Hunley T. E., Fogo A. Combined antagonism of endothelin A/B receptors links endothelin to vasoconstriction whereas angiotensin II effects fibrosis. Studies in chronic cyclosporine nephrotoxicity in rats. Transplantation. 1995 Jul 15;60(1):89–95. doi: 10.1097/00007890-199507150-00017. [DOI] [PubMed] [Google Scholar]
- Li S., Janosch P., Tanji M., Rosenfeld G. C., Waymire J. C., Mischak H., Kolch W., Sedivy J. M. Regulation of Raf-1 kinase activity by the 14-3-3 family of proteins. EMBO J. 1995 Feb 15;14(4):685–696. doi: 10.1002/j.1460-2075.1995.tb07047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister B., Lippoldt A., Bunnemann B., Inagami T., Ganten D., Fuxe K. Cellular expression of angiotensin type-1 receptor mRNA in the kidney. Kidney Int. 1993 Aug;44(2):331–336. doi: 10.1038/ki.1993.248. [DOI] [PubMed] [Google Scholar]
- Mihatsch M. J., Antonovych T., Bohman S. O., Habib R., Helmchen U., Noel L. H., Olsen S., Sibley R. K., Kemény E., Feutren G. Cyclosporin A nephropathy: standardization of the evaluation of kidney biopsies. Clin Nephrol. 1994 Jan;41(1):23–32. [PubMed] [Google Scholar]
- Myers B. D. Cyclosporine nephrotoxicity. Kidney Int. 1986 Dec;30(6):964–974. doi: 10.1038/ki.1986.280. [DOI] [PubMed] [Google Scholar]
- Nast C. C., Adler S. G., Artishevsky A., Kresser C. T., Ahmed K., Anderson P. S. Cyclosporine induces elevated procollagen alpha 1 (I) mRNA levels in the rat renal cortex. Kidney Int. 1991 Apr;39(4):631–638. doi: 10.1038/ki.1991.75. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Murray B. M. Renal dysfunction in animal models of cyclosporine toxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):155–159. [PubMed] [Google Scholar]
- Pimentel J. L., Jr, Sundell C. L., Wang S., Kopp J. B., Montero A., Martínez-Maldonado M. Role of angiotensin II in the expression and regulation of transforming growth factor-beta in obstructive nephropathy. Kidney Int. 1995 Oct;48(4):1233–1246. doi: 10.1038/ki.1995.407. [DOI] [PubMed] [Google Scholar]
- Ramirez-Bosca A., Kanitakis J., Thivolet J. Cyclosporin A exerts a cytostatic effect in vivo on human and murine epithelial cells. Cancer. 1990 Sep 1;66(5):936–940. doi: 10.1002/1097-0142(19900901)66:5<936::aid-cncr2820660521>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Chin J. E., Choi K. G., Gros P., Housman D. E., Fojo A., Shen D. W., Gottesman M. M., Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S., Greenfeld Z., Brezis M. Chronic cyclosporine-induced nephropathy in the rat. A medullary ray and inner stripe injury. Transplantation. 1990 Feb;49(2):445–452. doi: 10.1097/00007890-199002000-00041. [DOI] [PubMed] [Google Scholar]
- Rossi N. F., Churchill P. C., McDonald F. D., Ellis V. R. Mechanism of cyclosporine A-induced renal vasoconstriction in the rat. J Pharmacol Exp Ther. 1989 Sep;250(3):896–901. [PubMed] [Google Scholar]
- Ryffel B., Woerly G., Rodriguez C., Foxwell B. M. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for cyclosporine. J Recept Res. 1991;11(1-4):675–686. doi: 10.3109/10799899109066435. [DOI] [PubMed] [Google Scholar]
- Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993 Mar 25;268(9):6077–6080. [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schramm U., Fricker G., Wenger R., Miller D. S. P-glycoprotein-mediated secretion of a fluorescent cyclosporin analogue by teleost renal proximal tubules. Am J Physiol. 1995 Jan;268(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1995.268.1.F46. [DOI] [PubMed] [Google Scholar]
- Schuurhuis G. J., Broxterman H. J., Pinedo H. M., van Heijningen T. H., van Kalken C. K., Vermorken J. B., Spoelstra E. C., Lankelma J. The polyoxyethylene castor oil Cremophor EL modifies multidrug resistance. Br J Cancer. 1990 Oct;62(4):591–594. doi: 10.1038/bjc.1990.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihab F. S., Andoh T. F., Tanner A. M., Noble N. A., Border W. A., Franceschini N., Bennett W. M. Role of transforming growth factor-beta 1 in experimental chronic cyclosporine nephropathy. Kidney Int. 1996 Apr;49(4):1141–1151. doi: 10.1038/ki.1996.165. [DOI] [PubMed] [Google Scholar]
- Stillman I. E., Andoh T. F., Burdmann E. A., Bennett W. M., Rosen S. FK506 nephrotoxicity: morphologic and physiologic characterization of a rat model. Lab Invest. 1995 Dec;73(6):794–803. [PubMed] [Google Scholar]
- Stromskaya T. P., Grigorian I. A., Ossovskaya V. S., Rybalkina E. Y., Chumakov P. M., Kopnin B. P. Cell-specific effects of RAS oncogene and protein kinase C agonist TPA on P-glycoprotein function. FEBS Lett. 1995 Jul 17;368(2):373–376. doi: 10.1016/0014-5793(95)00662-s. [DOI] [PubMed] [Google Scholar]
- Sugawara I., Akiyama S., Scheper R. J., Itoyama S. Lung resistance protein (LRP) expression in human normal tissues in comparison with that of MDR1 and MRP. Cancer Lett. 1997 Jan 15;112(1):23–31. doi: 10.1016/S0304-3835(96)04542-9. [DOI] [PubMed] [Google Scholar]
- Tamai I., Safa A. R. Competitive interaction of cyclosporins with the Vinca alkaloid-binding site of P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1990 Sep 25;265(27):16509–16513. [PubMed] [Google Scholar]
- Twentyman P. R. Cyclosporins as drug resistance modifiers. Biochem Pharmacol. 1992 Jan 9;43(1):109–117. doi: 10.1016/0006-2952(92)90668-9. [DOI] [PubMed] [Google Scholar]
- Wei L. Y., Roepe P. D. Low external pH and osmotic shock increase the expression of human MDR protein. Biochemistry. 1994 Jun 14;33(23):7229–7238. doi: 10.1021/bi00189a027. [DOI] [PubMed] [Google Scholar]
- Young B. A., Burdmann E. A., Johnson R. J., Alpers C. E., Giachelli C. M., Eng E., Andoh T., Bennett W. M., Couser W. G. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995 Aug;48(2):439–448. doi: 10.1038/ki.1995.312. [DOI] [PubMed] [Google Scholar]
- Young B. A., Burdmann E. A., Johnson R. J., Andoh T., Bennett W. M., Couser W. G., Alpers C. E. Cyclosporine A induced arteriolopathy in a rat model of chronic cyclosporine nephropathy. Kidney Int. 1995 Aug;48(2):431–438. doi: 10.1038/ki.1995.311. [DOI] [PubMed] [Google Scholar]
- Zhang L., Sachs C. W., Fu H. W., Fine R. L., Casey P. J. Characterization of prenylcysteines that interact with P-glycoprotein and inhibit drug transport in tumor cells. J Biol Chem. 1995 Sep 29;270(39):22859–22865. doi: 10.1074/jbc.270.39.22859. [DOI] [PubMed] [Google Scholar]