Abstract
Stromelysin 3 (ST3) is a matrix metalloproteinase implicated in mammary carcinoma progression. To date, localization of ST3 expression in breast cancer by in situ hybridization and immunocytochemistry has shown that the expression of the enzyme is limited to only the stromal fibroblasts surrounding the cancer cells. We have immunostained a large group of ductal carcinoma in situ and invasive breast carcinomas using a monoclonal antibody (5ST-4A9) raised against the hemopexin-like domain of human ST3. We show that invasive lobular carcinomas express significantly less ST3 than invasive ductal carcinomas (IDCs) (P = 0.002). We also show, for the first time, that certain breast carcinoma cells that have undergone a degree of epithelial-to-mesenchymal transition, the so-called metaplastic carcinomas, can express ST3 mRNA and protein, which may in part explain the increased metastatic propensity seen in a number of these tumors. In addition, patients with IDC who had moderate to strong ST3 levels had significantly shorter disease-free survival than those with negative or weak ST3 levels (P = 0.02). Furthermore, in node-positive IDC patients, multivariate analysis revealed that ST3 level was a strong, independent prognostic parameter for disease-free survival (P = 0.005).
Full text
PDF


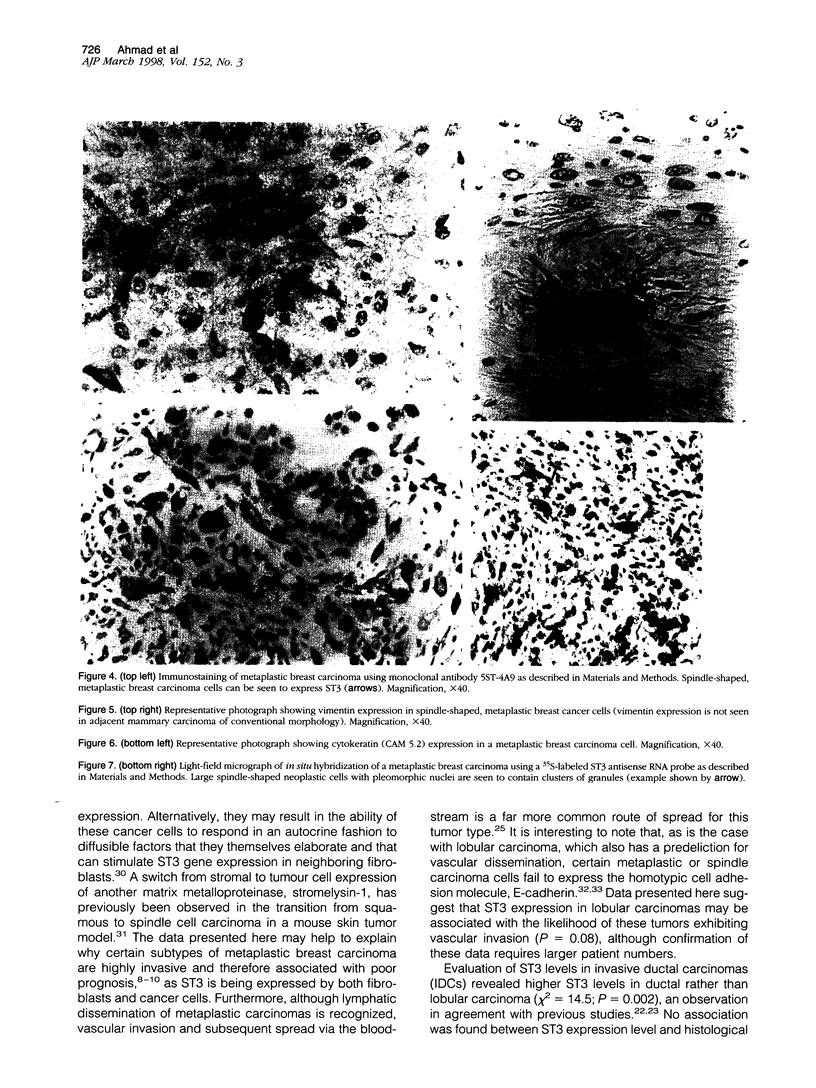


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad A., Marshall J. F., Basset P., Anglard P., Hart I. R. Modulation of human stromelysin 3 promoter activity and gene expression by human breast cancer cells. Int J Cancer. 1997 Oct 9;73(2):290–296. doi: 10.1002/(sici)1097-0215(19971009)73:2<290::aid-ijc21>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Chenard M. P., O'Siorain L., Shering S., Rouyer N., Lutz Y., Wolf C., Basset P., Bellocq J. P., Duffy M. J. High levels of stromelysin-3 correlate with poor prognosis in patients with breast carcinoma. Int J Cancer. 1996 Dec 20;69(6):448–451. doi: 10.1002/(SICI)1097-0215(19961220)69:6<448::AID-IJC5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Domagala W., Lasota J., Bartkowiak J., Weber K., Osborn M. Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki-67 growth fraction. Am J Pathol. 1990 Jan;136(1):219–227. [PMC free article] [PubMed] [Google Scholar]
- Domagala W., Woźniak L., Lasota J., Weber K., Osborn M. Vimentin is preferentially expressed in high-grade ductal and medullary, but not in lobular breast carcinomas. Am J Pathol. 1990 Nov;137(5):1059–1064. [PMC free article] [PubMed] [Google Scholar]
- Duffy M. J. The role of proteolytic enzymes in cancer invasion and metastasis. Clin Exp Metastasis. 1992 May;10(3):145–155. doi: 10.1007/BF00132746. [DOI] [PubMed] [Google Scholar]
- Engel G., Heselmeyer K., Auer G., Bäckdahl M., Eriksson E., Linder S. Correlation between stromelysin-3 mRNA level and outcome of human breast cancer. Int J Cancer. 1994 Sep 15;58(6):830–835. doi: 10.1002/ijc.2910580614. [DOI] [PubMed] [Google Scholar]
- Galea M. H., Blamey R. W., Elston C. E., Ellis I. O. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat. 1992;22(3):207–219. doi: 10.1007/BF01840834. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Trevor K. T. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol. 1997 Feb;150(2):483–495. [PMC free article] [PubMed] [Google Scholar]
- Holland R., Peterse J. L., Millis R. R., Eusebi V., Faverly D., van de Vijver M. J., Zafrani B. Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol. 1994 Aug;11(3):167–180. [PubMed] [Google Scholar]
- Hähnel E., Harvey J. M., Joyce R., Robbins P. D., Sterrett G. F., Hähnel R. Stromelysin-3 expression in breast cancer biopsies: clinico-pathological correlations. Int J Cancer. 1993 Nov 11;55(5):771–774. doi: 10.1002/ijc.2910550513. [DOI] [PubMed] [Google Scholar]
- King R. J., Barnes D. M., Hawkins R. A., Leake R. E., Maynard P. V., Roberts M. M. Measurement of oestradiol receptors by five institutions on common tissue samples. Br J Cancer. 1978 Sep;38(3):428–430. doi: 10.1038/bjc.1978.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisian L. M. The matrix-degrading metalloproteinases. Bioessays. 1992 Jul;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Muller D., Wolf C., Abecassis J., Millon R., Engelmann A., Bronner G., Rouyer N., Rio M. C., Eber M., Methlin G. Increased stromelysin 3 gene expression is associated with increased local invasiveness in head and neck squamous cell carcinomas. Cancer Res. 1993 Jan 1;53(1):165–169. [PubMed] [Google Scholar]
- Navarro P., Gómez M., Pizarro A., Gamallo C., Quintanilla M., Cano A. A role for the E-cadherin cell-cell adhesion molecule during tumor progression of mouse epidermal carcinogenesis. J Cell Biol. 1991 Oct;115(2):517–533. doi: 10.1083/jcb.115.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöel A. C., Lefebvre O., Maquoi E., VanHoorde L., Chenard M. P., Mareel M., Foidart J. M., Basset P., Rio M. C. Stromelysin-3 expression promotes tumor take in nude mice. J Clin Invest. 1996 Apr 15;97(8):1924–1930. doi: 10.1172/JCI118624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulyaeva H., Bueno J., Polette M., Birembaut P., Sato H., Seiki M., Thompson E. W. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis. 1997 Mar;15(2):111–120. doi: 10.1023/a:1018444609098. [DOI] [PubMed] [Google Scholar]
- Rouyer N., Wolf C., Chenard M. P., Rio M. C., Chambon P., Bellocq J. P., Basset P. Stromelysin-3 gene expression in human cancer: an overview. Invasion Metastasis. 1994;14(1-6):269–275. [PubMed] [Google Scholar]
- Santavicca M., Noel A., Chenard M. P., Lutz Y., Stoll I., Segain J. P., Rouyer N., Rio M. C., Wolf C., Belloco J. P. Characterization of monoclonal antibodies against stromelysin-3 and their use to evaluate stromelysin-3 levels in breast carcinoma by semi-quantitative immunohistochemistry. Int J Cancer. 1995 Oct 20;64(5):336–341. doi: 10.1002/ijc.2910640510. [DOI] [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Sommers C. L., Byers S. W., Thompson E. W., Torri J. A., Gelmann E. P. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31(2-3):325–335. doi: 10.1007/BF00666165. [DOI] [PubMed] [Google Scholar]
- Stoler A. B., Stenback F., Balmain A. The conversion of mouse skin squamous cell carcinomas to spindle cell carcinomas is a recessive event. J Cell Biol. 1993 Sep;122(5):1103–1117. doi: 10.1083/jcb.122.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K., Höyhtyä M., Salo T. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim Biophys Acta. 1987 Nov 25;907(3):191–217. doi: 10.1016/0304-419x(87)90006-0. [DOI] [PubMed] [Google Scholar]
- Wargotz E. S., Deos P. H., Norris H. J. Metaplastic carcinomas of the breast. II. Spindle cell carcinoma. Hum Pathol. 1989 Aug;20(8):732–740. doi: 10.1016/0046-8177(89)90065-8. [DOI] [PubMed] [Google Scholar]
- Wargotz E. S., Norris H. J. Metaplastic carcinomas of the breast. I. Matrix-producing carcinoma. Hum Pathol. 1989 Jul;20(7):628–635. doi: 10.1016/0046-8177(89)90149-4. [DOI] [PubMed] [Google Scholar]
- Wargotz E. S., Norris H. J. Metaplastic carcinomas of the breast. III. Carcinosarcoma. Cancer. 1989 Oct 1;64(7):1490–1499. doi: 10.1002/1097-0142(19891001)64:7<1490::aid-cncr2820640722>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wolf C., Rouyer N., Lutz Y., Adida C., Loriot M., Bellocq J. P., Chambon P., Basset P. Stromelysin 3 belongs to a subgroup of proteinases expressed in breast carcinoma fibroblastic cells and possibly implicated in tumor progression. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1843–1847. doi: 10.1073/pnas.90.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. H., McDonnell S., Portella G., Bowden G. T., Balmain A., Matrisian L. M. A switch from stromal to tumor cell expression of stromelysin-1 mRNA associated with the conversion of squamous to spindle carcinomas during mouse skin tumor progression. Mol Carcinog. 1994 Aug;10(4):207–215. doi: 10.1002/mc.2940100405. [DOI] [PubMed] [Google Scholar]
- Zandvliet D. W., Hanby A. M., Austin C. A., Marsh K. L., Clark I. B., Wright N. A., Poulsom R. Analysis of foetal expression sites of human type II DNA topoisomerase alpha and beta mRNAs by in situ hybridisation. Biochim Biophys Acta. 1996 Jun 7;1307(2):239–247. doi: 10.1016/0167-4781(96)00063-2. [DOI] [PubMed] [Google Scholar]