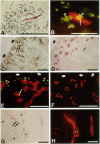
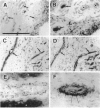
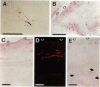
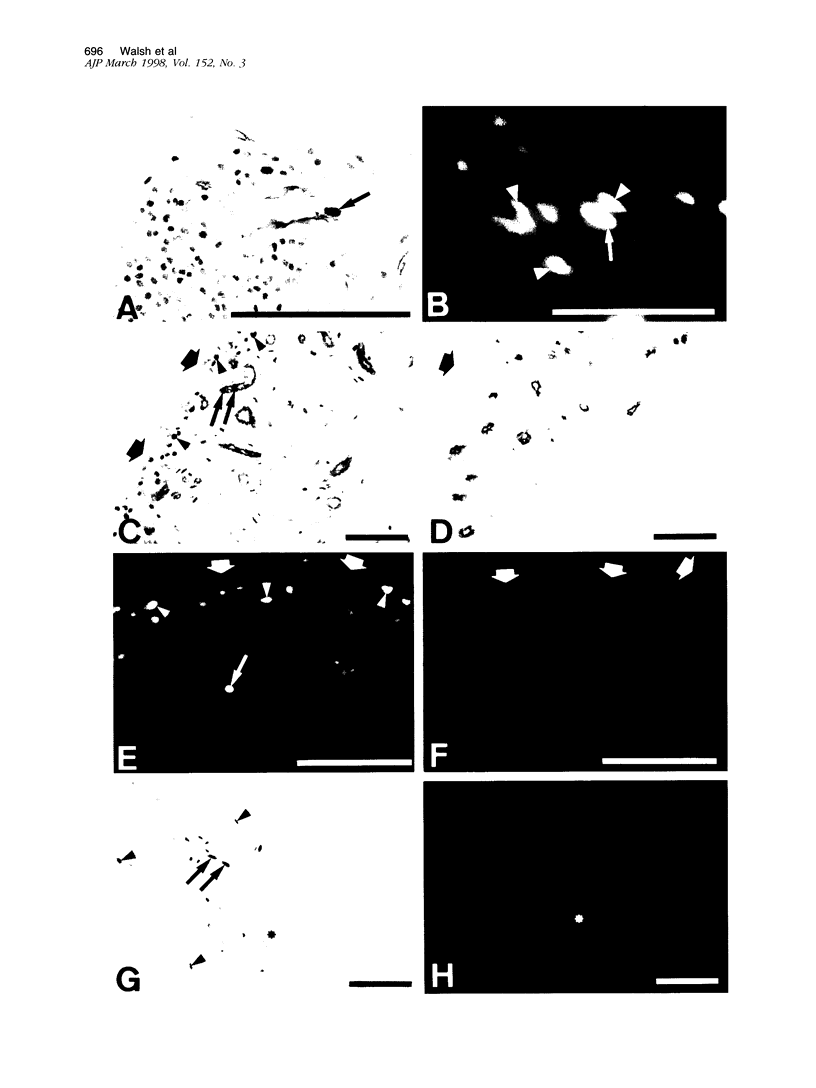
Abstract
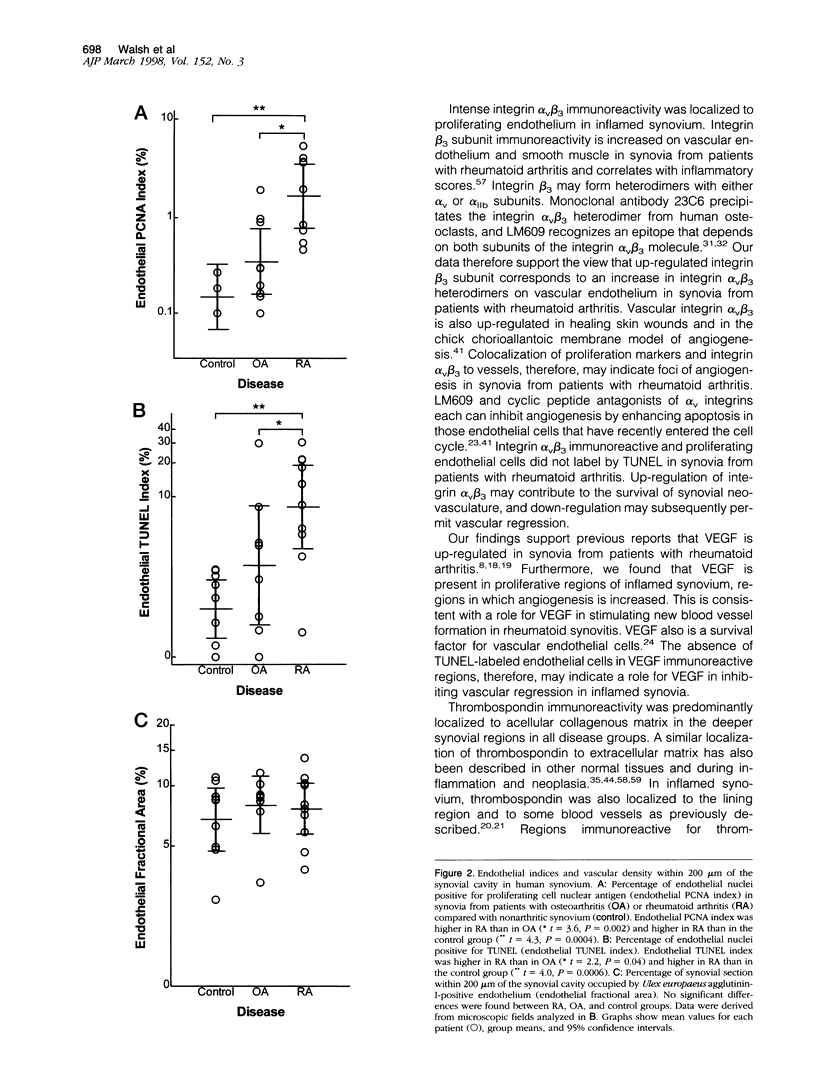
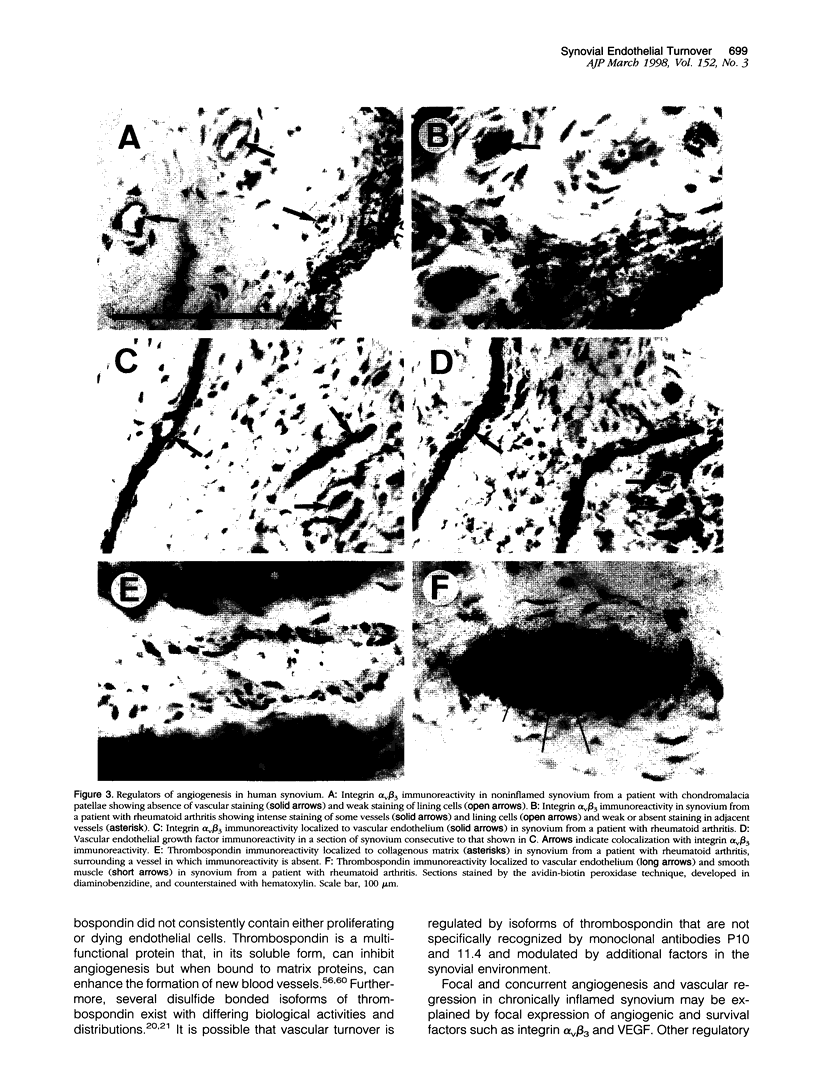
Angiogenesis and vascular insufficiency each may support the chronic synovial inflammation of rheumatoid arthritis. We have shown by quantitative immunohistochemistry and terminal uridyl deoxynucleotide nick end labeling that endothelial proliferation and cell death indices were each increased in synovia from patients with rheumatoid arthritis compared with osteoarthritic and noninflamed controls, whereas endothelial fractional areas did not differ significantly among disease groups. Markers of proliferation were associated with foci immunoreactive for vascular endothelial growth factor and integrin alpha(v)beta3, whereas cell death was observed in foci in which immunoreactivities for these factors were weak or absent. No association was found with thrombospondin immunoreactivity. The balance between angiogenesis and vascular regression in rheumatoid synovitis may be determined by the focal expression of angiogenic and endothelial survival factors. Increased endothelial cell turnover may contribute to microvascular dysfunction and thereby facilitate persistent synovitis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon T., Hemo I., Itin A., Pe'er J., Stone J., Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995 Oct;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Horton M. A., McGee J. O. New sites of cellular vitronectin receptor immunoreactivity detected with osteoclast-reacting monoclonal antibodies 13C2 and 23C6. Bone Miner. 1990 Jan;8(1):7–22. doi: 10.1016/0169-6009(91)90136-n. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Clark R. A., Cheresh D. A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994 Apr 22;264(5158):569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks P. C., Montgomery A. M., Rosenfeld M., Reisfeld R. A., Hu T., Klier G., Cheresh D. A. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994 Dec 30;79(7):1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Yeo K. T., Berse B., Yeo T. K., Senger D. R., Dvorak H. F., van de Water L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992 Nov 1;176(5):1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceponis A., Konttinen Y. T., MacKevicius Z., Solovieva S. A., Hukkanen M., Tamulaitiene M., Matulis A., Santavirta S. Aberrant vascularity and von Willebrand factor distribution in inflamed synovial membrane. J Rheumatol. 1996 Nov;23(11):1880–1886. [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Clezardin P., McGregor J. L., Lyon M., Clemetson K. J., Huppert J. Characterization of two murine monoclonal antibodies (P10, P12) directed against different determinants on human blood platelet thrombospondin. Eur J Biochem. 1986 Jan 2;154(1):95–102. doi: 10.1111/j.1432-1033.1986.tb09363.x. [DOI] [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Fava R. A., Olsen N. J., Spencer-Green G., Yeo K. T., Yeo T. K., Berse B., Jackman R. W., Senger D. R., Dvorak H. F., Brown L. F. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J Exp Med. 1994 Jul 1;180(1):341–346. doi: 10.1084/jem.180.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald O., Soden M., Yanni G., Robinson R., Bresnihan B. Morphometric analysis of blood vessels in synovial membranes obtained from clinically affected and unaffected knee joints of patients with rheumatoid arthritis. Ann Rheum Dis. 1991 Nov;50(11):792–796. doi: 10.1136/ard.50.11.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Falchuk K. H., Zeiger L. S., Sullivan A. L., Hebert C. L., Adams J. P., Decker J. L. A physiological approach to the assessment of disease activity in rheumatoid arthritis. J Clin Invest. 1971 Jun;50(6):1167–1180. doi: 10.1172/JCI106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Schmied M., Giegerich G., Breitschopf H., Hartung H. P., Toyka K. V., Lassmann H. Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest. 1994 Aug;71(2):219–225. [PubMed] [Google Scholar]
- Gorczyca W., Bigman K., Mittelman A., Ahmed T., Gong J., Melamed M. R., Darzynkiewicz Z. Induction of DNA strand breaks associated with apoptosis during treatment of leukemias. Leukemia. 1993 May;7(5):659–670. [PubMed] [Google Scholar]
- Grasl-Kraupp B., Ruttkay-Nedecky B., Koudelka H., Bukowska K., Bursch W., Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995 May;21(5):1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Recent insights into the pathogenesis of the proliferative lesion in rheumatoid arthritis. Arthritis Rheum. 1976 Jan-Feb;19(1):68–72. doi: 10.1002/art.1780190111. [DOI] [PubMed] [Google Scholar]
- Holthöfer H. Lectin binding sites in kidney. A comparative study of 14 animal species. J Histochem Cytochem. 1983 Apr;31(4):531–537. doi: 10.1177/31.4.6827083. [DOI] [PubMed] [Google Scholar]
- Johnson B. A., Haines G. K., Harlow L. A., Koch A. E. Adhesion molecule expression in human synovial tissue. Arthritis Rheum. 1993 Feb;36(2):137–146. doi: 10.1002/art.1780360203. [DOI] [PubMed] [Google Scholar]
- Kamel O. W., Franklin W. A., Ringus J. C., Meyer J. S. Thymidine labeling index and Ki-67 growth fraction in lesions of the breast. Am J Pathol. 1989 Jan;134(1):107–113. [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. The density and distribution of capillaries around a synovial cavity. Q J Exp Physiol. 1983 Oct;68(4):629–644. doi: 10.1113/expphysiol.1983.sp002753. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Friedman J., Burrows J. C., Haines G. K., Bouck N. P. Localization of the angiogenesis inhibitor thrombospondin in human synovial tissues. Pathobiology. 1993;61(1):1–6. doi: 10.1159/000163752. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Harlow L. A., Haines G. K., Amento E. P., Unemori E. N., Wong W. L., Pope R. M., Ferrara N. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J Immunol. 1994 Apr 15;152(8):4149–4156. [PubMed] [Google Scholar]
- Kuhn C., Mason R. J. Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol. 1995 Dec;147(6):1759–1769. [PMC free article] [PubMed] [Google Scholar]
- Lalor P. A., Mapp P. I., Hall P. A., Revell P. A. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7(5):183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Lang R., Lustig M., Francois F., Sellinger M., Plesken H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development. 1994 Dec;120(12):3395–3403. doi: 10.1242/dev.120.12.3395. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Hypoxia and acidosis in chronic inflammatory arthritis; relation to vascular supply and dynamic effusion pressure. J Rheumatol. 1990 May;17(5):579–582. [PubMed] [Google Scholar]
- Lund-Olesen K. Oxygen tension in synovial fluids. Arthritis Rheum. 1970 Nov-Dec;13(6):769–776. doi: 10.1002/art.1780130606. [DOI] [PubMed] [Google Scholar]
- Maeda K., Chung Y. S., Takatsuka S., Ogawa Y., Onoda N., Sawada T., Kato Y., Nitta A., Arimoto Y., Kondo Y. Tumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinoma. Br J Cancer. 1995 Aug;72(2):319–323. doi: 10.1038/bjc.1995.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias L. J., Gotis-Graham I., Underwood P. A., McNeil H. P., Hogg P. J. Identification of monoclonal antibodies that recognize different disulfide bonded forms of thrombospondin 1. Biochim Biophys Acta. 1996 Sep 5;1296(2):138–144. doi: 10.1016/0167-4838(96)00060-x. [DOI] [PubMed] [Google Scholar]
- McGregor B., Colon S., Mutin M., Chignier E., Zech P., McGregor J. Thrombospondin in human glomerulopathies. A marker of inflammation and early fibrosis. Am J Pathol. 1994 Jun;144(6):1281–1287. [PMC free article] [PubMed] [Google Scholar]
- Modlich U., Kaup F. J., Augustin H. G. Cyclic angiogenesis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest. 1996 Apr;74(4):771–780. [PubMed] [Google Scholar]
- Mohr W., Beneke G., Mohing W. Proliferation of synovial lining cells and fibroblasts. Ann Rheum Dis. 1975 Jun;34(3):219–224. doi: 10.1136/ard.34.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima M., Yoshino S., Ishiwata T., Asano G. Role of vascular endothelial growth factor in angiogenesis of rheumatoid arthritis. J Rheumatol. 1995 Sep;22(9):1624–1630. [PubMed] [Google Scholar]
- Nakajima T., Aono H., Hasunuma T., Yamamoto K., Shirai T., Hirohata K., Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995 Apr;38(4):485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Naughton D., Whelan M., Smith E. C., Williams R., Blake D. R., Grootveld M. An investigation of the abnormal metabolic status of synovial fluid from patients with rheumatoid arthritis by high field proton nuclear magnetic resonance spectroscopy. FEBS Lett. 1993 Feb 8;317(1-2):135–138. doi: 10.1016/0014-5793(93)81508-w. [DOI] [PubMed] [Google Scholar]
- Peacock D. J., Banquerigo M. L., Brahn E. A novel angiogenesis inhibitor suppresses rat adjuvant arthritis. Cell Immunol. 1995 Feb;160(2):178–184. doi: 10.1016/0008-8749(95)80025-e. [DOI] [PubMed] [Google Scholar]
- Peacock D. J., Banquerigo M. L., Brahn E. Angiogenesis inhibition suppresses collagen arthritis. J Exp Med. 1992 Apr 1;175(4):1135–1138. doi: 10.1084/jem.175.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Schilling J., Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989 Dec 1;8(12):3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z., Garcia C. H., O'Rourke L. M., Planck S. R., Kohli M., Rosenbaum J. T. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994 Feb;37(2):212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- Qu Z., Huang X. N., Ahmadi P., Andresevic J., Planck S. R., Hart C. E., Rosenbaum J. T. Expression of basic fibroblast growth factor in synovial tissue from patients with rheumatoid arthritis and degenerative joint disease. Lab Invest. 1995 Sep;73(3):339–346. [PubMed] [Google Scholar]
- Rastinejad F., Polverini P. J., Bouck N. P. Regulation of the activity of a new inhibitor of angiogenesis by a cancer suppressor gene. Cell. 1989 Feb 10;56(3):345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Robaye B., Dumont J. E. Phospholipase A2 activity is not involved in the tumor necrosis factor-triggered apoptotic DNA fragmentation in bovine aortic endothelial cells. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1312–1317. doi: 10.1016/0006-291x(92)91374-y. [DOI] [PubMed] [Google Scholar]
- Sanna P. P., Jirikowski G. F., Lewandowski G. A., Bloom F. E. Applications of DAPI cytochemistry to neurobiology. Biotech Histochem. 1992 Nov;67(6):346–350. doi: 10.3109/10520299209110047. [DOI] [PubMed] [Google Scholar]
- Schlingemann R. O., Rietveld F. J., de Waal R. M., Bradley N. J., Skene A. I., Davies A. J., Greaves M. F., Denekamp J., Ruiter D. J. Leukocyte antigen CD34 is expressed by a subset of cultured endothelial cells and on endothelial abluminal microprocesses in the tumor stroma. Lab Invest. 1990 Jun;62(6):690–696. [PubMed] [Google Scholar]
- Shai S. Y., Fishel R. S., Martin B. M., Berk B. C., Bernstein K. E. Bovine angiotensin converting enzyme cDNA cloning and regulation. Increased expression during endothelial cell growth arrest. Circ Res. 1992 Jun;70(6):1274–1281. doi: 10.1161/01.res.70.6.1274. [DOI] [PubMed] [Google Scholar]
- Sholley M. M., Ferguson G. P., Seibel H. R., Montour J. L., Wilson J. D. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest. 1984 Dec;51(6):624–634. [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Stevens C. R., Blake D. R., Merry P., Revell P. A., Levick J. R. A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum. 1991 Dec;34(12):1508–1513. doi: 10.1002/art.1780341206. [DOI] [PubMed] [Google Scholar]
- Sugiyama M., Tsukazaki T., Yonekura A., Matsuzaki S., Yamashita S., Iwasaki K. Localisation of apoptosis and expression of apoptosis related proteins in the synovium of patients with rheumatoid arthritis. Ann Rheum Dis. 1996 Jul;55(7):442–449. doi: 10.1136/ard.55.7.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Otsuka T., Matsui N., Asai K., Hirano T., Moriyama A., Isobe I., Eksioglu Y. Z., Matsukawa K., Kato T. Aberrant production of gliostatin/platelet-derived endothelial cell growth factor in rheumatoid synovium. Arthritis Rheum. 1994 May;37(5):662–672. doi: 10.1002/art.1780370509. [DOI] [PubMed] [Google Scholar]
- Vartanian R. K., Weidner N. Correlation of intratumoral endothelial cell proliferation with microvessel density (tumor angiogenesis) and tumor cell proliferation in breast carcinoma. Am J Pathol. 1994 Jun;144(6):1188–1194. [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Hu D. E., Mapp P. I., Polak J. M., Blake D. R., Fan T. P. Innervation and neurokinin receptors during angiogenesis in the rat sponge granuloma. Histochem J. 1996 Nov;28(11):759–769. doi: 10.1007/BF02272149. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Mapp P. I., Wharton J., Polak J. M., Blake D. R. Neuropeptide degrading enzymes in normal and inflamed human synovium. Am J Pathol. 1993 May;142(5):1610–1621. [PMC free article] [PubMed] [Google Scholar]
- Waseem N. H., Lane D. P. Monoclonal antibody analysis of the proliferating cell nuclear antigen (PCNA). Structural conservation and the detection of a nucleolar form. J Cell Sci. 1990 May;96(Pt 1):121–129. doi: 10.1242/jcs.96.1.121. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Wells A. F., Klareskog L., Lindblad S., Laurent T. C. Correlation between increased hyaluronan localized in arthritic synovium and the presence of proliferating cells. A role for macrophage-derived factors. Arthritis Rheum. 1992 Apr;35(4):391–396. doi: 10.1002/art.1780350405. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- Wong S. Y., Purdie A. T., Han P. Thrombospondin and other possible related matrix proteins in malignant and benign breast disease. An immunohistochemical study. Am J Pathol. 1992 Jun;140(6):1473–1482. [PMC free article] [PubMed] [Google Scholar]
- Zeymer U., Fishbein M. C., Forrester J. S., Cercek B. Proliferating cell nuclear antigen immunohistochemistry in rat aorta after balloon denudation. Comparison with thymidine and bromodeoxyuridine labeling. Am J Pathol. 1992 Sep;141(3):685–690. [PMC free article] [PubMed] [Google Scholar]