Abstract
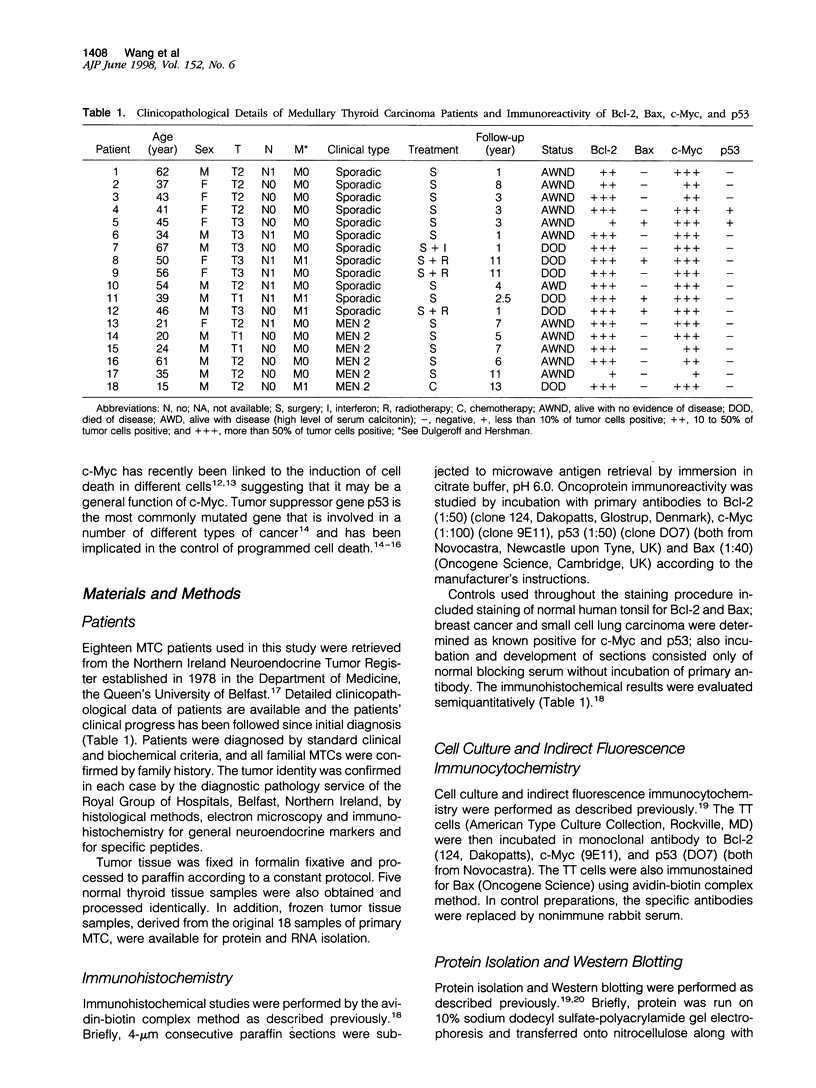
Medullary thyroid carcinoma (MTC) is a tumor of parafollicular cells of the thyroid gland. It has served as a useful experimental model for the study of tumor proliferation and differentiation. Although recent studies have identified the gene involved in familial forms of MTC, little is known about the molecular pathogenesis of the sporadic variants of this tumor. It has become increasingly clear that deregulation of programmed cell death is a critical component in multistep tumorigenesis. The present investigation was undertaken to determine whether similar molecular events occur in human MTC. Eighteen MTCs from 18 patients (including 12 sporadic and six familial cases and one metastatic lymph gland) and a MTC cell line (TT cells) were used in this study for detecting the expression of apoptosis-regulatory genes bcl-2, bax, c-myc, and p53. Immunohistochemical results showed that all MTC tumor samples displayed Bcl-2 and c-Myc immunoreactivity, whereas only 4 and 2 tumors showed a minority of cells positive for Bax and p53, respectively. Western and Northern blotting showed high levels of 26-kd Bcl-2 protein and bcl-2 transcript. The co-expression of Bcl-2 and c-Myc was also detected in the TT cells by indirect fluorescence immunohistochemistry and Western blotting. Moreover, Bcl-2 immunoreactivity was also found in C-cell hyperplasia from familial patients indicating that expression of this oncogene may represent an early event in the pathogenesis of MTC. The present study suggests that deregulation of programmed cell death may be a critical component in multistep tumorigenesis of MTC and that the frequent expression of the Bcl-2 oncoprotein in these tumors may contribute to their pathogenesis. The genetic complementation of simultaneously deregulated bcl-2 and c-myc may be implicated in the multistep tumorigenesis of human MTC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Basolo F., Pollina L., Fontanini G., Fiore L., Pacini F., Baldanzi A. Apoptosis and proliferation in thyroid carcinoma: correlation with bcl-2 and p53 protein expression. Br J Cancer. 1997;75(4):537–541. doi: 10.1038/bjc.1997.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Buchanan K. D., Johnston C. F., O'Hare M. M., Ardill J. E., Shaw C., Collins J. S., Watson R. G., Atkinson A. B., Hadden D. R., Kennedy T. L. Neuroendocrine tumors. A European view. Am J Med. 1986 Dec 22;81(6B):14–22. doi: 10.1016/0002-9343(86)90581-4. [DOI] [PubMed] [Google Scholar]
- Campos L., Rouault J. P., Sabido O., Oriol P., Roubi N., Vasselon C., Archimbaud E., Magaud J. P., Guyotat D. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood. 1993 Jun 1;81(11):3091–3096. [PubMed] [Google Scholar]
- Castle V. P., Heidelberger K. P., Bromberg J., Ou X., Dole M., Nuñez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993 Dec;143(6):1543–1550. [PMC free article] [PubMed] [Google Scholar]
- Chong G. C., Beahrs O. H., Sizemore G. W., Woolner L. H. Medullary carcinoma of the thyroid gland. Cancer. 1975 Mar;35(3):695–704. doi: 10.1002/1097-0142(197503)35:3<695::aid-cncr2820350323>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Dahia P. L., Aguiar R. C., Tsanaclis A. M., Bendit I., Bydlowski S. P., Abelin N. M., Toledo S. P. Molecular and immunohistochemical analysis of P53 in phaeochromocytoma. Br J Cancer. 1995 Nov;72(5):1211–1213. doi: 10.1038/bjc.1995.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulgeroff A. J., Hershman J. M. Medical therapy for differentiated thyroid carcinoma. Endocr Rev. 1994 Aug;15(4):500–515. doi: 10.1210/edrv-15-4-500. [DOI] [PubMed] [Google Scholar]
- Eng C. Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med. 1996 Sep 26;335(13):943–951. doi: 10.1056/NEJM199609263351307. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Fisher T. C., Milner A. E., Gregory C. D., Jackman A. L., Aherne G. W., Hartley J. A., Dive C., Hickman J. A. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993 Jul 15;53(14):3321–3326. [PubMed] [Google Scholar]
- Gottlieb J. A., Hill C. S., Jr, Ibanez M. L., Clark R. L. Chemotherapy of thyroid cancer. An evaluation of experience with 37 patients. Cancer. 1972 Sep;30(3):848–853. doi: 10.1002/1097-0142(197209)30:3<848::aid-cncr2820300336>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hickman J. A. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992 Sep;11(2):121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Husain M., Alsever R. N., Lock J. P., George W. F., Katz F. H. Failure of medullary carcinoma of the thyroid to respond to doxorubicin therapy. Horm Res. 1978;9(1):22–25. doi: 10.1159/000178893. [DOI] [PubMed] [Google Scholar]
- Jackson C. E., Tashjian A. H., Jr, Block M. A. Detection of medullary thyroid cancer by calcitonin assay in families. Ann Intern Med. 1973 Jun;78(6):845–852. doi: 10.7326/0003-4819-78-6-845. [DOI] [PubMed] [Google Scholar]
- Jiang S. X., Kameya T., Sato Y., Yanase N., Yoshimura H., Kodama T. Bcl-2 protein expression in lung cancer and close correlation with neuroendocrine differentiation. Am J Pathol. 1996 Mar;148(3):837–846. [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Oursler M. J., Schroeder M. J., Eberhardt N. L. Transforming growth factor-beta 1 induces growth inhibition of a human medullary thyroid carcinoma cell line despite an increase in steady state c-myc messenger ribonucleic acid levels. Endocrinology. 1994 Nov;135(5):1887–1893. doi: 10.1210/endo.135.5.7956909. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992 Aug 15;80(4):879–886. [PubMed] [Google Scholar]
- Korsmeyer S. J., Shutter J. R., Veis D. J., Merry D. E., Oltvai Z. N. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin Cancer Biol. 1993 Dec;4(6):327–332. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Langdon W. Y., Harris A. W., Cory S., Adams J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell. 1986 Oct 10;47(1):11–18. doi: 10.1016/0092-8674(86)90361-2. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Lohmann D. R., Fesseler B., Pütz B., Reich U., Böhm J., Präuer H., Wünsch P. H., Höfler H. Infrequent mutations of the p53 gene in pulmonary carcinoid tumors. Cancer Res. 1993 Dec 1;53(23):5797–5801. [PubMed] [Google Scholar]
- Lohmann D. R., Funk A., Niedermeyer H. P., Häupel S., Höfler H. Identification of p53 gene mutations in gastrointestinal and pancreatic carcinoids by nonradioisotopic SSCA. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64(5):293–296. doi: 10.1007/BF02915125. [DOI] [PubMed] [Google Scholar]
- Loke S. L., Neckers L. M., Schwab G., Jaffe E. S. c-myc protein in normal tissue. Effects of fixation on its apparent subcellular distribution. Am J Pathol. 1988 Apr;131(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- McDonnell T. J. Cell division versus cell death: a functional model of multistep neoplasia. Mol Carcinog. 1993;8(4):209–213. doi: 10.1002/mc.2940080402. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Korsmeyer S. J. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991 Jan 17;349(6306):254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin B. D., de Bustros A. C., Mabry M., Baylin S. B. The molecular biology of medullary thyroid carcinoma. A model for cancer development and progression. JAMA. 1989 Jun 2;261(21):3130–3135. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Meister L., Tanaka S., Cuddy M., Yum S., Geyer C., Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991 Dec 15;51(24):6529–6538. [PubMed] [Google Scholar]
- Royds J. A., Sharrard R. M., Wagner B., Polacarz S. V. Cellular localisation of c-myc product in human colorectal epithelial neoplasia. J Pathol. 1992 Mar;166(3):225–233. doi: 10.1002/path.1711660304. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Bath M. L., Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wagner A. J., Small M. B., Hay N. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol. 1993 Apr;13(4):2432–2440. doi: 10.1128/mcb.13.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. G., Barros D'Sa A. A., Johnston C. F., Buchanan K. D. Oncogene expression in carotid body tumors. Cancer. 1996 Jun 15;77(12):2581–2587. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2581::AID-CNCR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wang D. G., Johnston C. F., Anderson N., Sloan J. M., Buchanan K. D. Overexpression of the tumour suppressor gene p53 is not implicated in neuroendocrine tumour carcinogenesis. J Pathol. 1995 Apr;175(4):397–401. doi: 10.1002/path.1711750406. [DOI] [PubMed] [Google Scholar]
- Wang D. G., Johnston C. F., Atkinson A. B., Heaney A. P., Mirakhur M., Buchanan K. D. Expression of bcl-2 oncoprotein in pituitary tumours: comparison with c-myc. J Clin Pathol. 1996 Oct;49(10):795–797. doi: 10.1136/jcp.49.10.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. G., Johnston C. F., Barros D'Sa A. A., Buchanan K. D. Expression of apoptosis-suppressing gene bcl-2 in human carotid body tumours. J Pathol. 1997 Oct;183(2):218–221. doi: 10.1002/(SICI)1096-9896(199710)183:2<218::AID-PATH910>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Wang D. G., Johnston C. F., Buchanan K. D. Oncogene expression in gastroenteropancreatic neuroendocrine tumors: implications for pathogenesis. Cancer. 1997 Aug 15;80(4):668–675. [PubMed] [Google Scholar]
- Wang D. G., Johnston C. F., Marley J. J., Phenix K. V., Atkinson A. B., Russell C. F., Buchanan K. D. Expression of the apoptosis-suppressing gene BCL-2 in pheochromocytoma is associated with the expression of C-MYC. J Clin Endocrinol Metab. 1997 Jun;82(6):1949–1952. doi: 10.1210/jcem.82.6.4008. [DOI] [PubMed] [Google Scholar]
- Wang Wenliang, Johansson Henry, Bergholm Ulla, Wilander Erik, Grimelius Lars. Apoptosis and Expression of the Proto-Oncogenes bcl-2 and p53 and the Proliferation Factor Ki-67 in Human Medullary Thyroid Carcinoma. Endocr Pathol. 1996 Spring;7(1):37–45. doi: 10.1007/BF02739913. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wolfe H. J., Melvin K. E., Cervi-Skinner S. J., Saadi A. A., Juliar J. F., Jackson C. E., Tashjian A. H., Jr C-cell hyperplasia preceding medullary thyroid carcinoma. N Engl J Med. 1973 Aug 30;289(9):437–441. doi: 10.1056/NEJM197308302890901. [DOI] [PubMed] [Google Scholar]
- Yana I., Nakamura T., Shin E., Karakawa K., Kurahashi H., Kurita Y., Kobayashi T., Mori T., Nishisho I., Takai S. Inactivation of the p53 gene is not required for tumorigenesis of medullary thyroid carcinoma or pheochromocytoma. Jpn J Cancer Res. 1992 Nov;83(11):1113–1116. doi: 10.1111/j.1349-7006.1992.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C., Knudson C. M., Korsmeyer S. J., Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997 Feb 13;385(6617):637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K., Iwahana H., Fukuda A., Sano T., Saito S., Itakura M. Role of p53 mutations in endocrine tumorigenesis: mutation detection by polymerase chain reaction-single strand conformation polymorphism. Cancer Res. 1992 Sep 15;52(18):5061–5064. [PubMed] [Google Scholar]





