Abstract
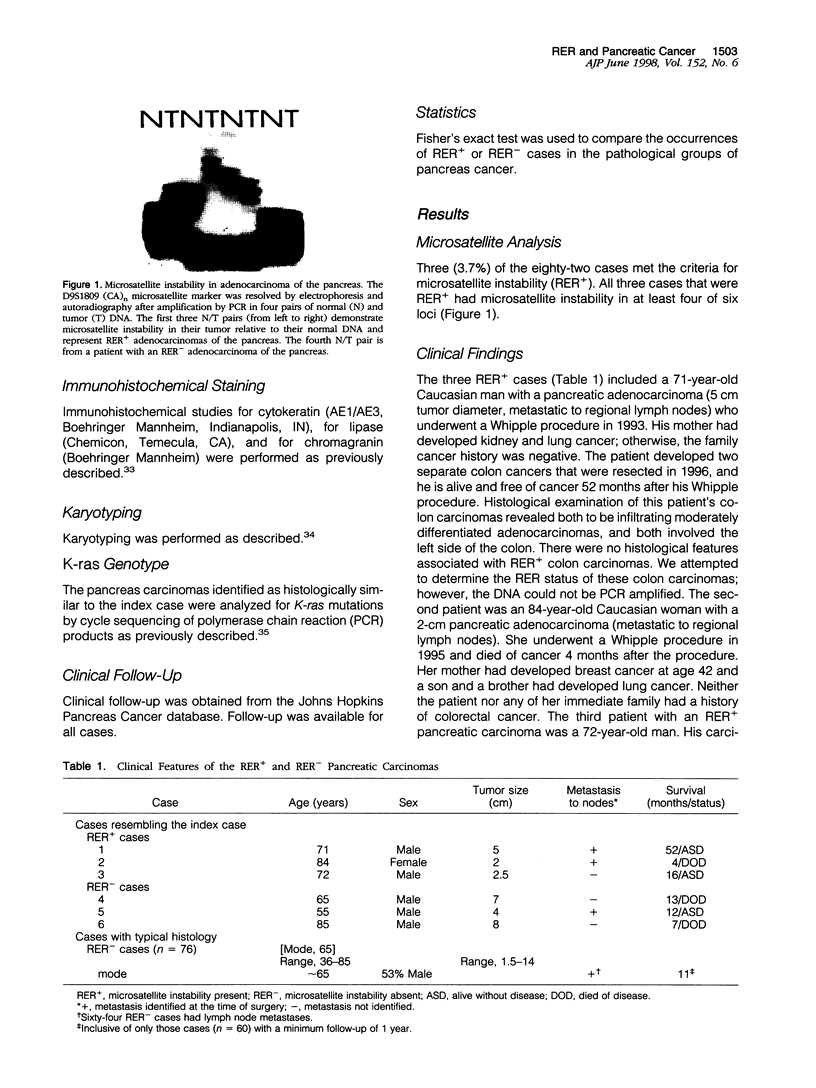
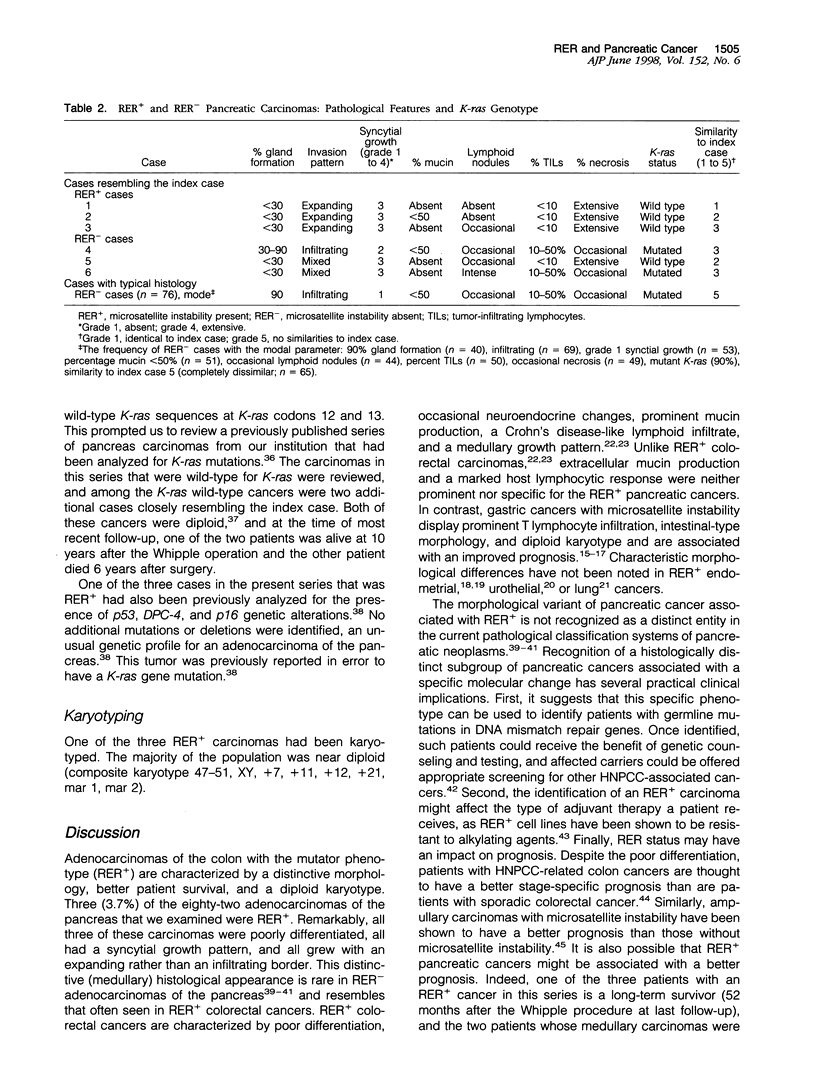
The clinical and pathological features of carcinomas of the pancreas with DNA replication errors (RER+) have not been characterized. Eighty-two xenografted carcinomas of the pancreas were screened for DNA replication errors using polymerase chain reaction amplification of microsatellite markers. Cases with microsatellite instability in at least two markers of a minimum of five tested were considered RER+. RER status was correlated with histological appearance, karyotype of the carcinomas when available, K-ras mutational status, and patient outcome. Three (3.7%) of the eighty-two carcinomas were RER+. In contrast to typical gland-forming adenocarcinomas of the pancreas, all three RER+ carcinomas were poorly differentiated and had expanding borders and a prominent syncytial growth pattern. Neither a Crohn's-like lymphoid infiltrate nor extracellular mucin production were prominent. Ductal adenocarcinomas of the pancreas typically contain a mutant K-ras gene, yet all three RER+ carcinomas had wild-type K-ras. One of the three RER+ carcinomas was karyotyped and showed a near diploid pattern. All three of the RER+ tumors were removed via Whipple resection. One of the three patients is free of disease 16 months after pancreaticoduodenectomy, one is alive and free of tumor at 52 months but developed two colon carcinomas during this period, and the third died of pancreatic cancer at 4 months. None of the three patients had a family history of colorectal carcinoma. A review of the K-ras wild-type carcinomas in a previously characterized series of pancreatic carcinomas with known K-ras mutational status identified two additional cancers with poor differentiation, a syncytial growth pattern, and pushing borders. Both of the cancers were diploid and both patients were longterm survivors (over 5 years). The inclusion of such patients in previous prognostic studies of pancreas cancer may explain the failure of histological grade to be a predictor of prognosis. These data suggest that DNA replication errors occur in a small percentage of resected carcinomas of the pancreas and that wild-type K-ras gene status and a medullary phenotype characterized by poor differentiation, and expanding pattern of invasion, and syncytial growth should suggest the possibility of DNA replication errors in carcinomas of the pancreas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Achille A., Biasi M. O., Zamboni G., Bogina G., Iacono C., Talamini G., Capella G., Scarpa A. Cancers of the papilla of vater: mutator phenotype is associated with good prognosis. Clin Cancer Res. 1997 Oct;3(10):1841–1847. [PubMed] [Google Scholar]
- Akiyama Y., Sato H., Yamada T., Nagasaki H., Tsuchiya A., Abe R., Yuasa Y. Germ-line mutation of the hMSH6/GTBP gene in an atypical hereditary nonpolyposis colorectal cancer kindred. Cancer Res. 1997 Sep 15;57(18):3920–3923. [PubMed] [Google Scholar]
- Albano W. A., Recabaren J. A., Lynch H. T., Campbell A. S., Mailliard J. A., Organ C. H., Lynch J. F., Kimberling W. J. Natural history of hereditary cancer of the breast and colon. Cancer. 1982 Jul 15;50(2):360–363. doi: 10.1002/1097-0142(19820715)50:2<360::aid-cncr2820500233>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Allison D. C., Bose K. K., Hruban R. H., Piantadosi S., Dooley W. C., Boitnott J. K., Cameron J. L. Pancreatic cancer cell DNA content correlates with long-term survival after pancreatoduodenectomy. Ann Surg. 1991 Dec;214(6):648–656. doi: 10.1097/00000658-199112000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Burke W., Petersen G., Lynch P., Botkin J., Daly M., Garber J., Kahn M. J., McTiernan A., Offit K., Thomson E. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA. 1997 Mar 19;277(11):915–919. [PubMed] [Google Scholar]
- Burks R. T., Kessis T. D., Cho K. R., Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994 Apr;9(4):1163–1166. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., Hruban R. H., Redston M. S., Yeo C. J., Kern S. E. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994 Jul 1;54(13):3568–3573. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Chung Y. J., Song J. M., Lee J. Y., Jung Y. T., Seo E. J., Choi S. W., Rhyu M. G. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 1996 Oct 15;56(20):4662–4665. [PubMed] [Google Scholar]
- Cubilla A. L., Fitzgerald P. J. Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res. 1975 Aug;35(8):2234–2248. [PubMed] [Google Scholar]
- DiGiuseppe J. A., Redston M. S., Yeo C. J., Kern S. E., Hruban R. H. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol. 1995 Oct;147(4):884–888. [PMC free article] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fujii H., Shimada T. Isolation and characterization of cDNA clones derived from the divergently transcribed gene in the region upstream from the human dihydrofolate reductase gene. J Biol Chem. 1989 Jun 15;264(17):10057–10064. [PubMed] [Google Scholar]
- Graham D. M., Appelman H. D. Crohn's-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990 May;3(3):332–335. [PubMed] [Google Scholar]
- Griffin C. A., Hruban R. H., Morsberger L. A., Ellingham T., Long P. P., Jaffee E. M., Hauda K. M., Bohlander S. K., Yeo C. J. Consistent chromosome abnormalities in adenocarcinoma of the pancreas. Cancer Res. 1995 Jun 1;55(11):2394–2399. [PubMed] [Google Scholar]
- Hahn S. A., Seymour A. B., Hoque A. T., Schutte M., da Costa L. T., Redston M. S., Caldas C., Weinstein C. L., Fischer A., Yeo C. J. Allelotype of pancreatic adenocarcinoma using xenograft enrichment. Cancer Res. 1995 Oct 15;55(20):4670–4675. [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Love S. B., Northover J. M. A new prognostic classification of rectal cancer. Lancet. 1987 Jun 6;1(8545):1303–1306. doi: 10.1016/s0140-6736(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Smyrk T. C., Stewart S. M., Lane M. R., Lanspa S. J., Lynch H. T. Pathology of hereditary non-polyposis colorectal cancer. Anticancer Res. 1994 Jul-Aug;14(4B):1631–1634. [PubMed] [Google Scholar]
- Kim H., Jen J., Vogelstein B., Hamilton S. R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994 Jul;145(1):148–156. [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Sagae S., Kudo R., Saito H., Koi S., Nakamura Y. Microsatellite instability in endometrial carcinomas: frequent replication errors in tumors of early onset and/or of poorly differentiated type. Genes Chromosomes Cancer. 1995 Oct;14(2):128–132. doi: 10.1002/gcc.2870140207. [DOI] [PubMed] [Google Scholar]
- Koi M., Umar A., Chauhan D. P., Cherian S. P., Carethers J. M., Kunkel T. A., Boland C. R. Human chromosome 3 corrects mismatch repair deficiency and microsatellite instability and reduces N-methyl-N'-nitro-N-nitrosoguanidine tolerance in colon tumor cells with homozygous hMLH1 mutation. Cancer Res. 1994 Aug 15;54(16):4308–4312. [PubMed] [Google Scholar]
- Kouri M., Laasonen A., Mecklin J. P., Järvinen H., Franssila K., Pyrhönen S. Diploid predominance in hereditary nonpolyposis colorectal carcinoma evaluated by flow cytometry. Cancer. 1990 Apr 15;65(8):1825–1829. doi: 10.1002/1097-0142(19900415)65:8<1825::aid-cncr2820650827>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Losi L., Fante R., Di Gregorio C., Aisoni M. L., Lanza G., Maestri I., Roncucci L., Pedroni M., Ponz de Leon M. Biologic characterization of hereditary non-polyposis colorectal cancer. Nuclear ploidy, AgNOR count, microvessel distribution, oncogene expression, and grade-related parameters. Am J Clin Pathol. 1995 Mar;103(3):265–270. doi: 10.1093/ajcp/103.3.265. [DOI] [PubMed] [Google Scholar]
- Lothe R. A., Peltomäki P., Meling G. I., Aaltonen L. A., Nyström-Lahti M., Pylkkänen L., Heimdal K., Andersen T. I., Møller P., Rognum T. O. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993 Dec 15;53(24):5849–5852. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T., Lynch J. F. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch Syndrome). Int J Cancer. 1996 Feb 20;69(1):38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Markowitz S., Wang J., Myeroff L., Parsons R., Sun L., Lutterbaugh J., Fan R. S., Zborowska E., Kinzler K. W., Vogelstein B. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995 Jun 2;268(5215):1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- Mecklin J. P., Järvinen H. J. Clinical features of colorectal carcinoma in cancer family syndrome. Dis Colon Rectum. 1986 Mar;29(3):160–164. doi: 10.1007/BF02555012. [DOI] [PubMed] [Google Scholar]
- Merlo A., Mabry M., Gabrielson E., Vollmer R., Baylin S. B., Sidransky D. Frequent microsatellite instability in primary small cell lung cancer. Cancer Res. 1994 Apr 15;54(8):2098–2101. [PubMed] [Google Scholar]
- Olschwang S., Hamelin R., Laurent-Puig P., Thuille B., De Rycke Y., Li Y. J., Muzeau F., Girodet J., Salmon R. J., Thomas G. Alternative genetic pathways in colorectal carcinogenesis. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12122–12127. doi: 10.1073/pnas.94.22.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Lothe R. A., Aaltonen L. A., Pylkkänen L., Nyström-Lahti M., Seruca R., David L., Holm R., Ryberg D., Haugen A. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993 Dec 15;53(24):5853–5855. [PubMed] [Google Scholar]
- Rozenblum E., Schutte M., Goggins M., Hahn S. A., Panzer S., Zahurak M., Goodman S. N., Sohn T. A., Hruban R. H., Yeo C. J. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997 May 1;57(9):1731–1734. [PubMed] [Google Scholar]
- Seruca R., Santos N. R., David L., Constância M., Barroca H., Carneiro F., Seixas M., Peltomäki P., Lothe R., Sobrinho-Simões M. Sporadic gastric carcinomas with microsatellite instability display a particular clinicopathologic profile. Int J Cancer. 1995 Feb 20;64(1):32–36. doi: 10.1002/ijc.2910640108. [DOI] [PubMed] [Google Scholar]
- Smit V. T., Boot A. J., Smits A. M., Fleuren G. J., Cornelisse C. J., Bos J. L. KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res. 1988 Aug 25;16(16):7773–7782. doi: 10.1093/nar/16.16.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T., Fujimori T., Maeda S., Yamamoto M., Saitoh Y. The role of Ras mutation in pancreatic cancer, precancerous lesions, and chronic pancreatitis. Int J Pancreatol. 1993 Dec;14(3):237–244. doi: 10.1007/BF02784932. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Uchida T., Wang C., Wada C., Iwamura M., Egawa S., Koshiba K. Microsatellite instability in transitional cell carcinoma of the urinary tract and its relationship to clinicopathological variables and smoking. Int J Cancer. 1996 Apr 22;69(2):142–145. doi: 10.1002/(SICI)1097-0215(19960422)69:2<142::AID-IJC13>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Wiggers T., Arends J. W., Schutte B., Volovics L., Bosman F. T. A multivariate analysis of pathologic prognostic indicators in large bowel cancer. Cancer. 1988 Jan 15;61(2):386–395. doi: 10.1002/1097-0142(19880115)61:2<386::aid-cncr2820610231>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- dos Santos N. R., Seruca R., Constância M., Seixas M., Sobrinho-Simões M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996 Jan;110(1):38–44. doi: 10.1053/gast.1996.v110.pm8536886. [DOI] [PubMed] [Google Scholar]




