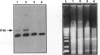
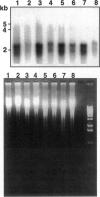
Abstract
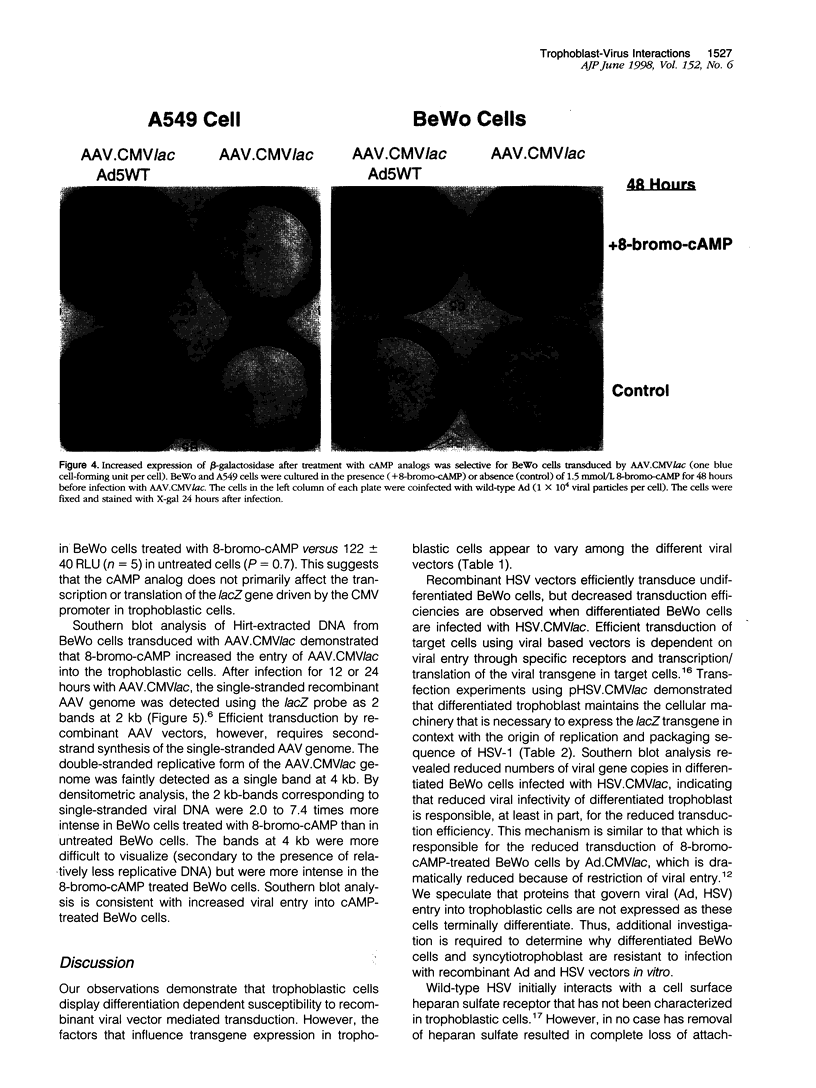
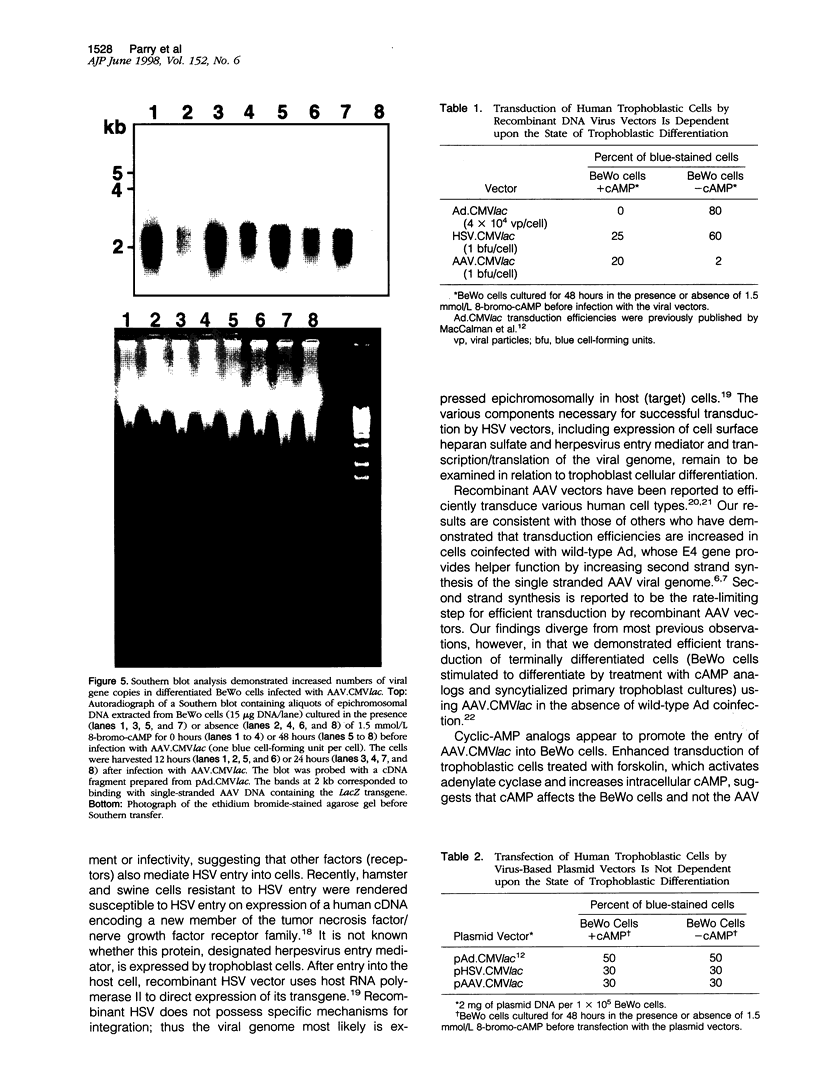
We investigated the transfer of the lacZ reporter gene into human trophoblastic cells using herpes simplex virus and adeno-associated virus vectors. We used an established choriocarcinoma cell line (BeWo cells) that can be induced to terminally differentiate after treatment with cyclic-AMP. Our results demonstrate that transduction of trophoblastic cells by the herpes simplex virus vector, HSV.CMVlac, and the adeno-associated virus vector, AAV.CMVlac, is affected by cellular differentiation. Treatment of BeWo cells with cyclic-AMP reduced transduction by HSV.CMVlac but increased transduction by the AAV vector. In contrast, when BeWo cells were transfected with herpes simplex virus and adeno-associated virus plasmids, lacZ expression was not affected by treatment with cyclic-AMP. Southern blot analysis demonstrated 2.75 times less herpes simplex virus DNA in cyclic-AMP treated BeWo cells, but 2.0 to 7.4 times more adeno-associated virus DNA in treated cells. We conclude that inefficient transduction of differentiated trophoblastic cells with HSV.CMVlac is because of diminished viral entry, whereas cellular differentiation is associated with increased entry of AAV.CMVlac. These observations suggest that adeno-associated virus vectors may be used to modify trophoblast function and study placental physiology. Additionally, trophoblast differentiation leads to alterations in the mechanisms of virus uptake that may affect maternal-to-fetus transmission.
Full text
PDF


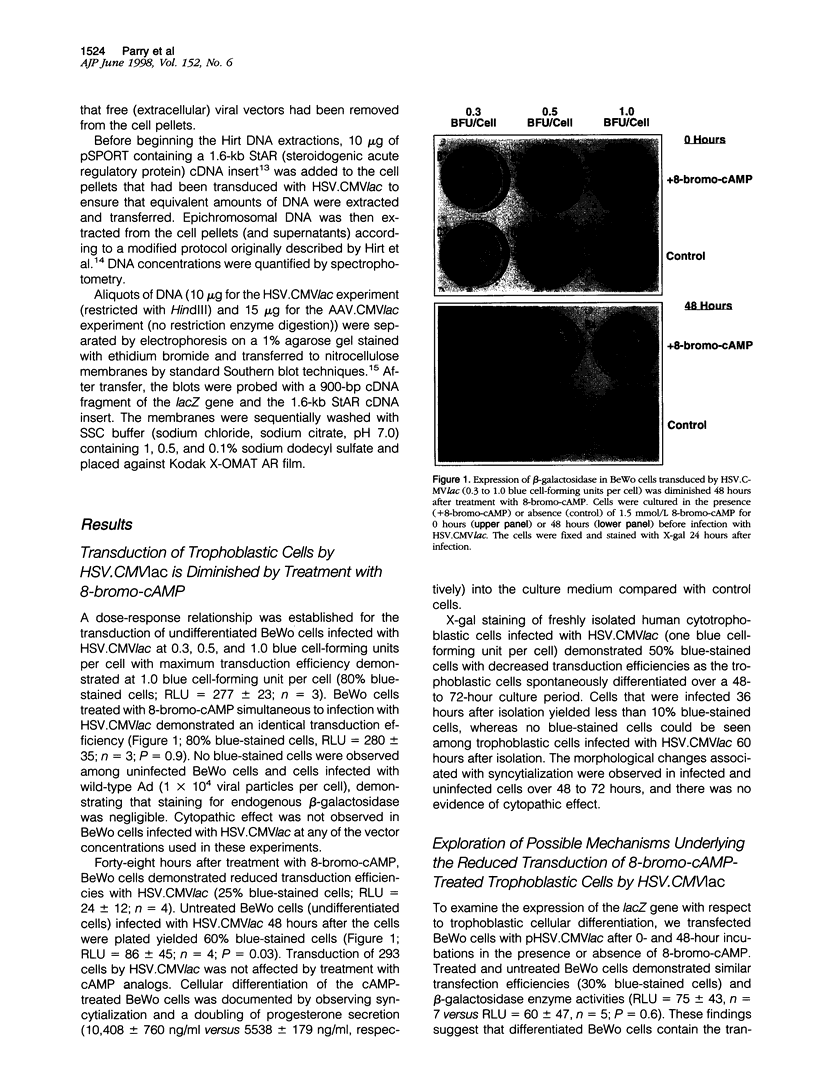

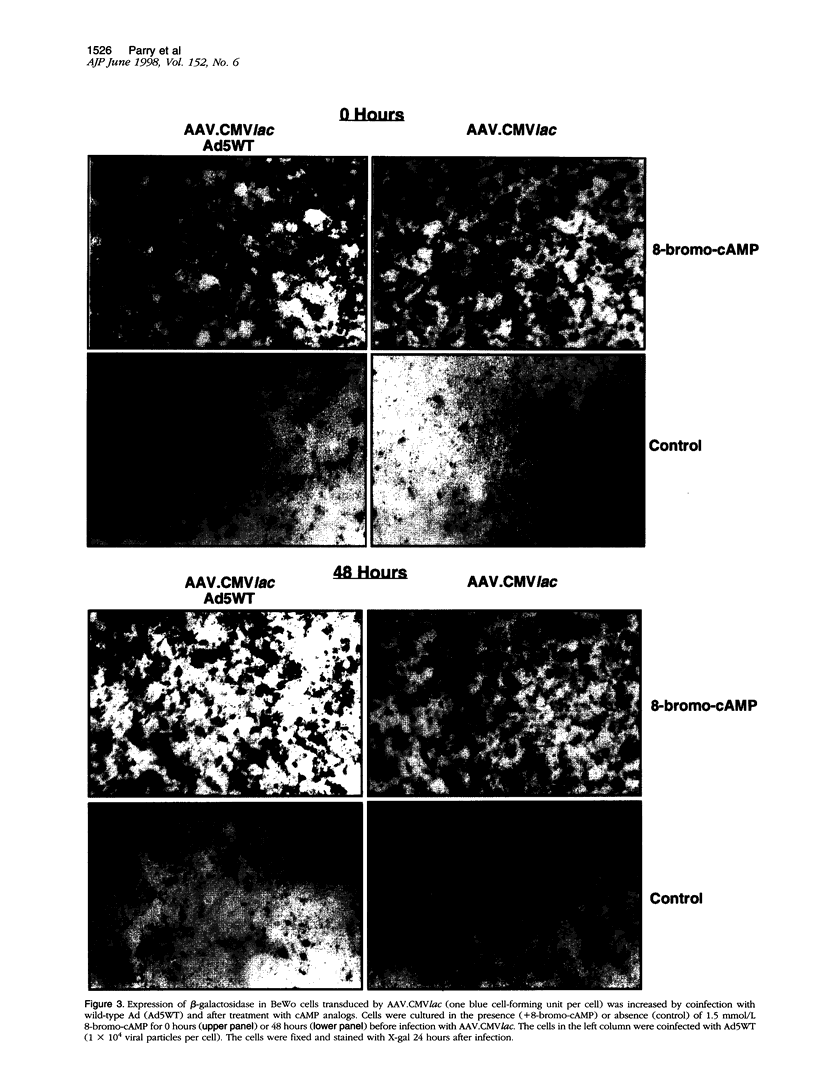

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns K. I., Linden R. M. The cryptic life style of adeno-associated virus. Bioessays. 1995 Mar;17(3):237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- Crystal R. G. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995 Oct 20;270(5235):404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- Ferrari F. K., Samulski T., Shenk T., Samulski R. J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996 May;70(5):3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K. J., Gao G. P., Weitzman M. D., DeMatteo R., Burda J. F., Wilson J. M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996 Jan;70(1):520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. R., Carter B. J. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995 Aug;2(6):357–362. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hutto C., Arvin A., Jacobs R., Steele R., Stagno S., Lyrene R., Willett L., Powell D., Andersen R., Werthammer J. Intrauterine herpes simplex virus infections. J Pediatr. 1987 Jan;110(1):97–101. doi: 10.1016/s0022-3476(87)80298-6. [DOI] [PubMed] [Google Scholar]
- Kaplan C. The placenta and viral infections. Semin Diagn Pathol. 1993 Aug;10(3):232–250. [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus amplicon: effect of size on replication of constructed defective genomes containing eucaryotic DNA sequences. J Virol. 1984 Sep;51(3):595–603. doi: 10.1128/jvi.51.3.595-603.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., McNally M. M., Okarma T. B., Lerch L. B. Adeno-associated virus: a vector system for efficient introduction and integration of DNA into a variety of mammalian cell types. Mol Cell Biol. 1988 Oct;8(10):3988–3996. doi: 10.1128/mcb.8.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Gupta S., Federoff H. Ex vivo hepatic gene transfer in mouse using a defective herpes simplex virus-1 vector. Hepatology. 1995 Mar;21(3):752–759. [PubMed] [Google Scholar]
- MacCalman C. D., Furth E. E., Omigbodun A., Kozarsky K. F., Coutifaris C., Strauss J. F., 3rd Transduction of human trophoblast cells by recombinant adenoviruses is differentiation dependent. Biol Reprod. 1996 Mar;54(3):682–691. doi: 10.1095/biolreprod54.3.682. [DOI] [PubMed] [Google Scholar]
- Malhomme O., Dutheil N., Rabreau M., Armbruster-Moraes E., Schlehofer J. R., Dupressoir T. Human genital tissues containing DNA of adeno-associated virus lack DNA sequences of the helper viruses adenovirus, herpes simplex virus or cytomegalovirus but frequently contain human papillomavirus DNA. J Gen Virol. 1997 Aug;78(Pt 8):1957–1962. doi: 10.1099/0022-1317-78-8-1957. [DOI] [PubMed] [Google Scholar]
- Montgomery R. I., Warner M. S., Lum B. J., Spear P. G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996 Nov 1;87(3):427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- Nørskov-Lauritsen N., Aboagye-Mathisen G., Juhl C. B., Petersen P. M., Zachar V., Ebbesen P. Herpes simplex virus infection of cultured human term trophoblast. J Med Virol. 1992 Mar;36(3):162–166. doi: 10.1002/jmv.1890360303. [DOI] [PubMed] [Google Scholar]
- Ringler G. E., Strauss J. F., 3rd In vitro systems for the study of human placental endocrine function. Endocr Rev. 1990 Feb;11(1):105–123. doi: 10.1210/edrv-11-1-105. [DOI] [PubMed] [Google Scholar]
- Rolling F., Samulski R. J. AAV as a viral vector for human gene therapy. Generation of recombinant virus. Mol Biotechnol. 1995 Feb;3(1):9–15. doi: 10.1007/BF02821330. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Miller A. D., Alexander I. E. Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8915–8919. doi: 10.1073/pnas.91.19.8915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T., Holt J. A., Driscoll D., Strauss J. F., 3rd, Lin D., Miller W. L., Patterson D., Clancy K. P., Hart I. M., Clark B. J. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4778–4782. doi: 10.1073/pnas.92.11.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiasch E., Rabreau M., Geletneky K., Laruë-Charlus S., Severin F., Becker N., Schlehofer J. R. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994 Oct;44(2):215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- Wittmaack F. M., Gåfvels M. E., Bronner M., Matsuo H., McCrae K. R., Tomaszewski J. E., Robinson S. L., Strickland D. K., Strauss J. F., 3rd Localization and regulation of the human very low density lipoprotein/apolipoprotein-E receptor: trophoblast expression predicts a role for the receptor in placental lipid transport. Endocrinology. 1995 Jan;136(1):340–348. doi: 10.1210/endo.136.1.7828550. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]