Abstract
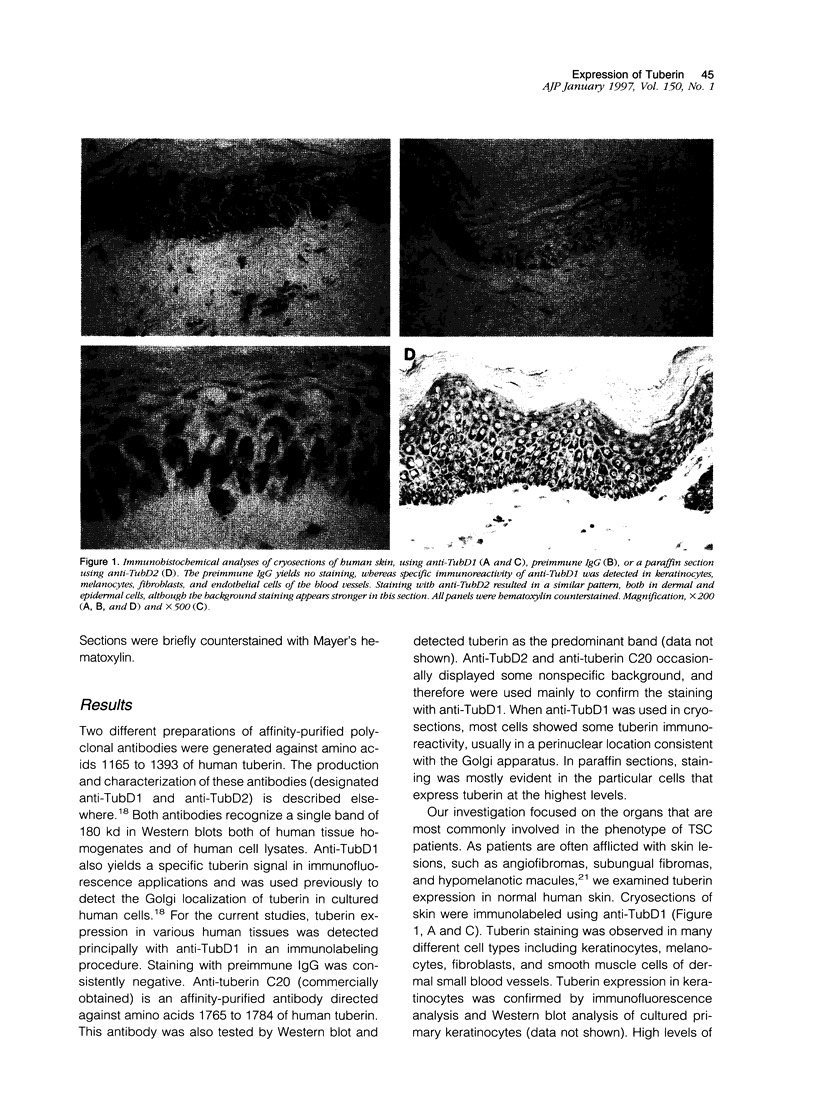
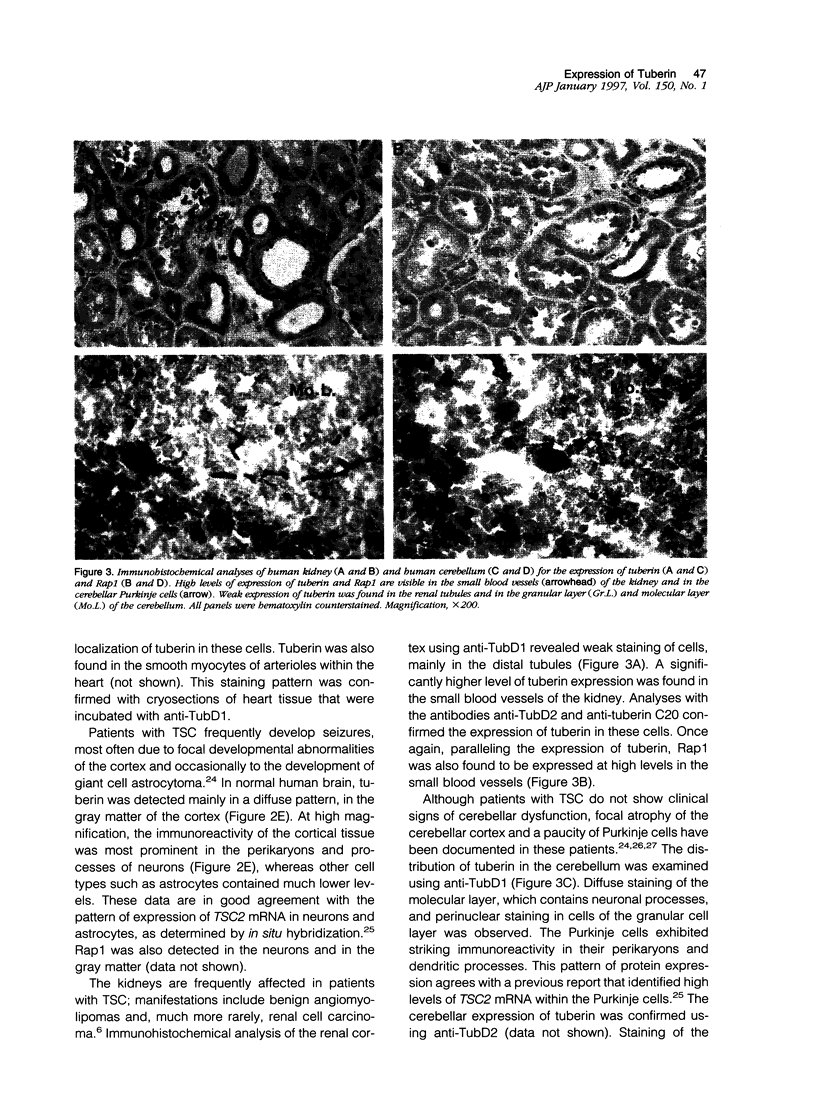
The tuberous sclerosis-2 (TSC2) gene is linked to tuberous sclerosis (TSC), a dominantly inherited genetic syndrome in which inactivation of the normal TSC2 allele is associated with the development of mostly benign tumors and focal dysplasias. TSC2 encodes the protein tuberin, which is a widely expressed 180-kd polypeptide that exhibits specific GTPase activating activity toward Rap1 in vitro and co-localizes with Rap1 in cultured cells. In this study, we have performed immunohistochemical analyses, using affinity-purified anti-tuberin antibodies, to study the distribution of tuberin in a panel of normal human organs that are commonly affected by TSC. Cryosections indicated that tuberin is widely expressed at low levels. More intense staining of tuberin, in the cryosections and in paraffin sections, was observed in the small blood vessels of many organs, including the kidney, skin, and adrenal gland. High levels of tuberin were also detected in cortical neurons and cerebellar Purkinje cells. These findings imply that loss-of-function mutations in TSC2 might lead to the development of highly vascularized tumors, subcortical tubers, and focal atrophy of the cerebellar cortex, which are features commonly associated with TSC. Moreover, Rap1 was also found to be highly expressed in many of the same cells that contained high levels of tuberin, suggesting a functional interaction between tuberin and Rap1 in these tissues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein J., Robbins T. O. Renal involvement in tuberous sclerosis. Ann N Y Acad Sci. 1991;615:36–49. doi: 10.1111/j.1749-6632.1991.tb37746.x. [DOI] [PubMed] [Google Scholar]
- Béranger F., Goud B., Tavitian A., de Gunzburg J. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1606–1610. doi: 10.1073/pnas.88.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonara C., Longa L., Grosso E., Mazzucco G., Borrone C., Garrè M. L., Brisigotti M., Filippi G., Scabar A., Giannotti A. Apparent preferential loss of heterozygosity at TSC2 over TSC1 chromosomal region in tuberous sclerosis hamartomas. Genes Chromosomes Cancer. 1996 Jan;15(1):18–25. doi: 10.1002/(SICI)1098-2264(199601)15:1<18::AID-GCC3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Cook S. J., Rubinfeld B., Albert I., McCormick F. RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J. 1993 Sep;12(9):3475–3485. doi: 10.1002/j.1460-2075.1993.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Geist R. T., Gutmann D. H. The tuberous sclerosis 2 gene is expressed at high levels in the cerebellum and developing spinal cord. Cell Growth Differ. 1995 Nov;6(11):1477–1483. [PubMed] [Google Scholar]
- Green A. J., Sepp T., Yates J. R. Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet. 1996 Feb;97(2):240–243. doi: 10.1007/BF02265273. [DOI] [PubMed] [Google Scholar]
- Henske E. P., Neumann H. P., Scheithauer B. W., Herbst E. W., Short M. P., Kwiatkowski D. J. Loss of heterozygosity in the tuberous sclerosis (TSC2) region of chromosome band 16p13 occurs in sporadic as well as TSC-associated renal angiomyolipomas. Genes Chromosomes Cancer. 1995 Aug;13(4):295–298. doi: 10.1002/gcc.2870130411. [DOI] [PubMed] [Google Scholar]
- Janssen B., Sampson J., van der Est M., Deelen W., Verhoef S., Daniels I., Hesseling A., Brook-Carter P., Nellist M., Lindhout D. Refined localization of TSC1 by combined analysis of 9q34 and 16p13 data in 14 tuberous sclerosis families. Hum Genet. 1994 Oct;94(4):437–440. doi: 10.1007/BF00201608. [DOI] [PubMed] [Google Scholar]
- Kim H., Kerr A., Morehouse H. The association between tuberous sclerosis and insulinoma. AJNR Am J Neuroradiol. 1995 Aug;16(7):1543–1544. [PMC free article] [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Short M. P. Tuberous sclerosis. Arch Dermatol. 1994 Mar;130(3):348–354. [PubMed] [Google Scholar]
- Lie J. T. Cardiac, pulmonary, and vascular involvements in tuberous sclerosis. Ann N Y Acad Sci. 1991;615:58–70. doi: 10.1111/j.1749-6632.1991.tb37748.x. [DOI] [PubMed] [Google Scholar]
- Northrup H., Wheless J. W., Bertin T. K., Lewis R. A. Variability of expression in tuberous sclerosis. J Med Genet. 1993 Jan;30(1):41–43. doi: 10.1136/jmg.30.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T., Shimizu K., Yamamori B., Kuroda S., Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J Biol Chem. 1996 Jan 19;271(3):1258–1261. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- Osborne J. P., Fryer A., Webb D. Epidemiology of tuberous sclerosis. Ann N Y Acad Sci. 1991;615:125–127. doi: 10.1111/j.1749-6632.1991.tb37754.x. [DOI] [PubMed] [Google Scholar]
- Pizon V., Lerosey I., Chardin P., Tavitian A. Nucleotide sequence of a human cDNA encoding a ras-related protein (rap1B). Nucleic Acids Res. 1988 Aug 11;16(15):7719–7719. doi: 10.1093/nar/16.15.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson E. P., Jr Pathology of tuberous sclerosis. Neuropathologic aspects. Ann N Y Acad Sci. 1991;615:128–139. doi: 10.1111/j.1749-6632.1991.tb37755.x. [DOI] [PubMed] [Google Scholar]
- Shepherd C. W., Gomez M. R., Lie J. T., Crowson C. S. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc. 1991 Aug;66(8):792–796. doi: 10.1016/s0025-6196(12)61196-3. [DOI] [PubMed] [Google Scholar]
- Short M. P., Richardson E. P., Jr, Haines J. L., Kwiatkowski D. J. Clinical, neuropathological and genetic aspects of the tuberous sclerosis complex. Brain Pathol. 1995 Apr;5(2):173–179. doi: 10.1111/j.1750-3639.1995.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Watson G. H. Cardiac rhabdomyomas in tuberous sclerosis. Ann N Y Acad Sci. 1991;615:50–57. doi: 10.1111/j.1749-6632.1991.tb37747.x. [DOI] [PubMed] [Google Scholar]
- Wiederholt W. C., Gomez M. R., Kurland L. T. Incidence and prevalence of tuberous sclerosis in Rochester, Minnesota, 1950 through 1982. Neurology. 1985 Apr;35(4):600–603. doi: 10.1212/wnl.35.4.600. [DOI] [PubMed] [Google Scholar]
- Wienecke R., König A., DeClue J. E. Identification of tuberin, the tuberous sclerosis-2 product. Tuberin possesses specific Rap1GAP activity. J Biol Chem. 1995 Jul 7;270(27):16409–16414. doi: 10.1074/jbc.270.27.16409. [DOI] [PubMed] [Google Scholar]
- Wienecke R., Maize J. C., Jr, Shoarinejad F., Vass W. C., Reed J., Bonifacino J. S., Resau J. H., de Gunzburg J., Yeung R. S., DeClue J. E. Co-localization of the TSC2 product tuberin with its target Rap1 in the Golgi apparatus. Oncogene. 1996 Sep 5;13(5):913–923. [PubMed] [Google Scholar]
- Xiao G. H., Jin F., Yeung R. S. Identification of tuberous sclerosis 2 messenger RNA splice variants that are conserved and differentially expressed in rat and human tissues. Cell Growth Differ. 1995 Sep;6(9):1185–1191. [PubMed] [Google Scholar]
- Xu L., Sterner C., Maheshwar M. M., Wilson P. J., Nellist M., Short P. M., Haines J. L., Sampson J. R., Ramesh V. Alternative splicing of the tuberous sclerosis 2 (TSC2) gene in human and mouse tissues. Genomics. 1995 Jun 10;27(3):475–480. doi: 10.1006/geno.1995.1079. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Kawata M., Miura Y., Musha T., Sasaki T., Kikuchi A., Takai Y. Microinjection of smg/rap1/Krev-1 p21 into Swiss 3T3 cells induces DNA synthesis and morphological changes. Mol Cell Biol. 1992 Aug;12(8):3407–3414. doi: 10.1128/mcb.12.8.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvulunov A., Esterly N. B. Neurocutaneous syndromes associated with pigmentary skin lesions. J Am Acad Dermatol. 1995 Jun;32(6):915–937. doi: 10.1016/0190-9622(95)91325-4. [DOI] [PubMed] [Google Scholar]