Abstract
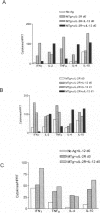
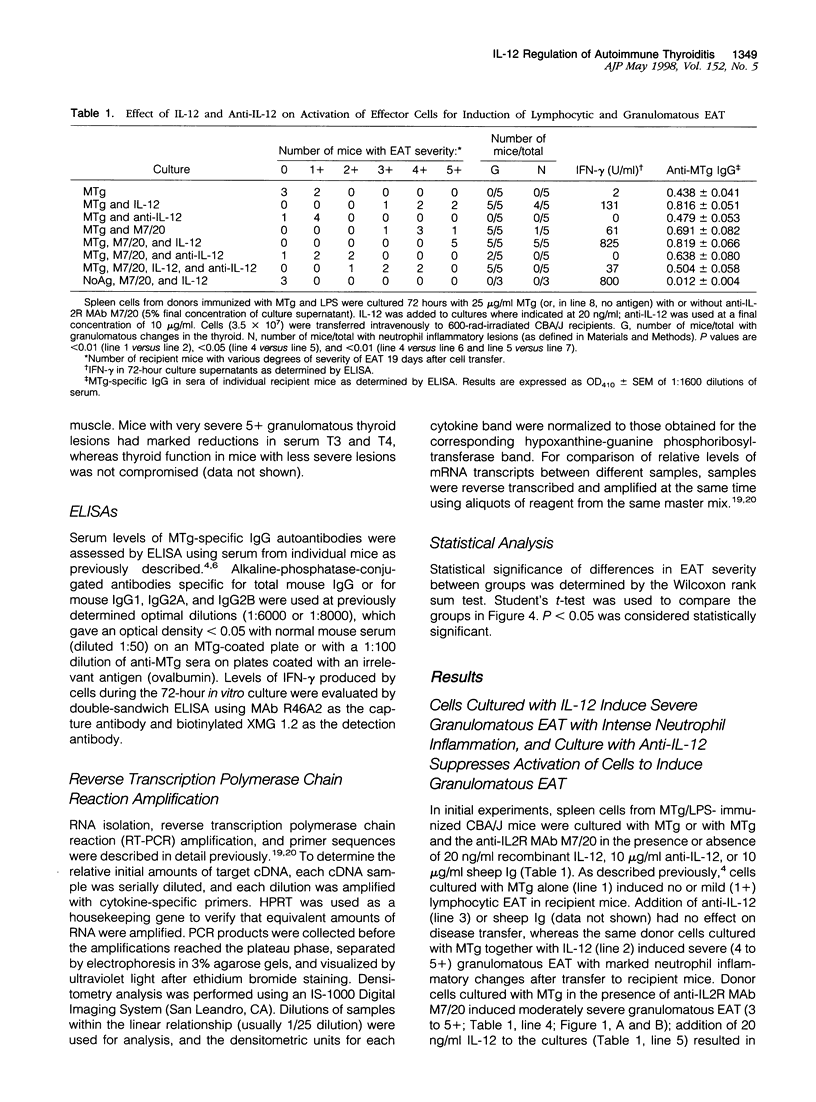
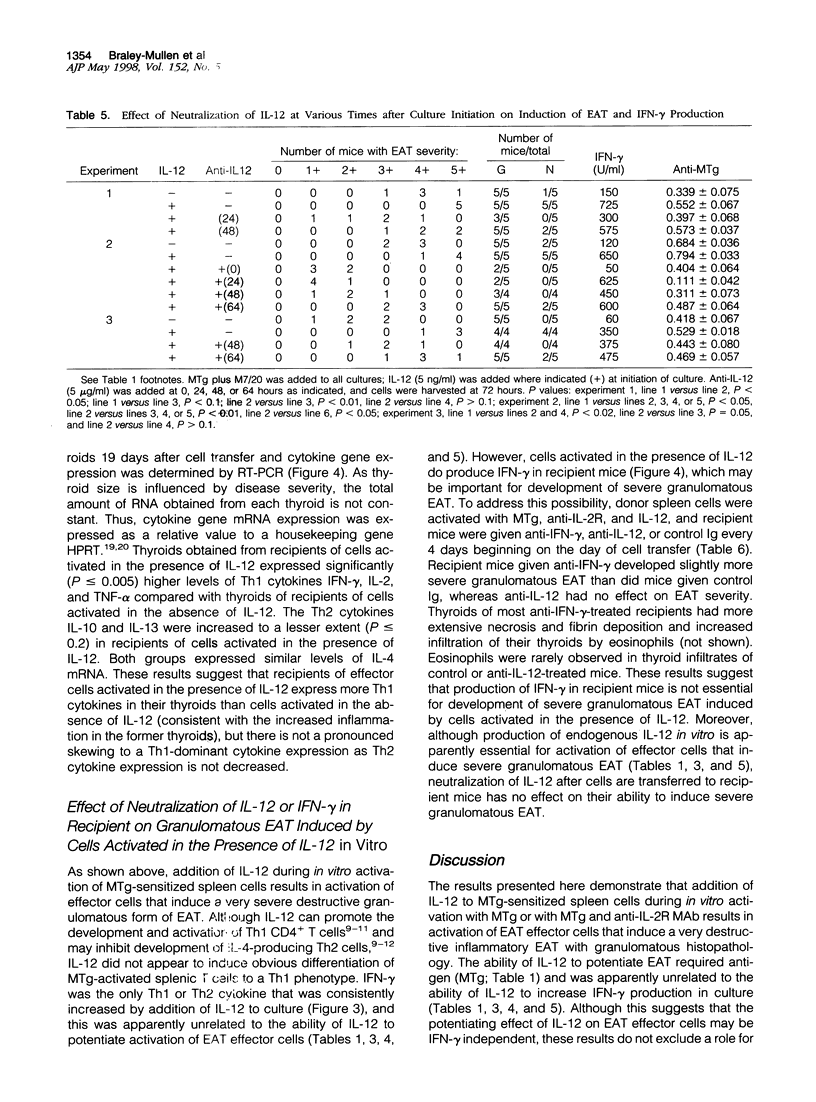
Granulomatous inflammatory lesions are a major histopathological feature of a wide spectrum of human infectious and autoimmune diseases. Experimental autoimmune thyroiditis (EAT) with granulomatous histopathological features can be induced by mouse thyroglobulin (MTg)-sensitized spleen cells activated in vitro with MTg and anti-interleukin-2 receptor (anti-IL-2R), anti-IL-2, or anti-interferon-gamma (anti-IFN-gamma) monoclonal antibody (MAb). These studies suggested that IFN-gamma-producing T cells requiring IL-2 for growth may negatively regulate activation of granulomatous EAT effector cells. As IL-12 promotes activation of IFN-gamma-producing Th1 cells, the present study was undertaken to determine the role of IL-12 in activation of effector cells for granulomatous EAT. MTg-sensitized cells activated in vitro with MTg, anti-IL2R MAb, and IL-12 induced severe, destructive granulomatous thyroiditis with neutrophil inflammation, fibrin deposition, and necrosis. Many glands ultimately underwent atrophy and became fibrotic; some also showed fibrinoid necrosis and a mixed inflammatory cell infiltration of blood vessel walls indicative of a necrotizing vasculitis. Induction of severe granulomatous EAT by IL-12 required MTg in vitro and was unrelated to the IL-12-induced increase in IFN-gamma production. IL-12 markedly increased IFN-gamma production but did not induce a shift to a Th1-dominant phenotype, as other Th1 and Th2 cytokines were generally unaffected and both Th1 and Th2 cytokines were expressed in recipient thyroids. Addition of IL-12 or neutralization by anti-IL-12 at various times indicated that IL-12 exerted its primary effects in the final 24 hours of the 72-hour culture and was not required in recipient mice. Cells cultured with anti-IL-12, MTg, and anti-IL2R MAb transferred mild lymphocytic EAT but little or no granulomatous EAT. Thus, IL-12 profoundly regulates the in vitro activation of effector cells that induce histologically distinct autoimmune inflammatory lesions in the thyroid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Murphy K. M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Abdi K., Herrmann S. H. CTL generation in the presence of IL-4 is inhibited by free p40: evidence for early and late IL-12 function. J Immunol. 1997 Oct 1;159(7):3148–3155. [PubMed] [Google Scholar]
- Adorini L., Gregori S., Magram J., Trembleau S. The role of IL-12 in the pathogenesis of Th1 cell-mediated autoimmune diseases. Ann N Y Acad Sci. 1996 Oct 31;795:208–215. doi: 10.1111/j.1749-6632.1996.tb52670.x. [DOI] [PubMed] [Google Scholar]
- Bliss J., Van Cleave V., Murray K., Wiencis A., Ketchum M., Maylor R., Haire T., Resmini C., Abbas A. K., Wolf S. F. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996 Feb 1;156(3):887–894. [PubMed] [Google Scholar]
- Braley-Mullen H., Johnson M., Sharp G. C., Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985 Jun;93(1):132–143. doi: 10.1016/0008-8749(85)90394-6. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H., McMurray R. W., Sharp G. C., Kyriakos M. Regulation of the induction and resolution of granulomatous experimental autoimmune thyroiditis in mice by CD8+ T cells. Cell Immunol. 1994 Feb;153(2):492–504. doi: 10.1006/cimm.1994.1045. [DOI] [PubMed] [Google Scholar]
- Braley-Mullen H., Sharp G. C., Bickel J. T., Kyriakos M. Induction of severe granulomatous experimental autoimmune thyroiditis in mice by effector cells activated in the presence of anti-interleukin 2 receptor antibody. J Exp Med. 1991 Apr 1;173(4):899–912. doi: 10.1084/jem.173.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunda M. J. Interleukin-12. J Leukoc Biol. 1994 Feb;55(2):280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- Charreire J. Immune mechanisms in autoimmune thyroiditis. Adv Immunol. 1989;46:263–334. doi: 10.1016/s0065-2776(08)60656-2. [DOI] [PubMed] [Google Scholar]
- Chehimi J., Valiante N. M., D'Andrea A., Rengaraju M., Rosado Z., Kobayashi M., Perussia B., Wolf S. F., Starr S. E., Trinchieri G. Enhancing effect of natural killer cell stimulatory factor (NKSF/interleukin-12) on cell-mediated cytotoxicity against tumor-derived and virus-infected cells. Eur J Immunol. 1993 Aug;23(8):1826–1830. doi: 10.1002/eji.1830230814. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai B. B., Quinn P. M., Wolitzky A. G., Mongini P. K., Chizzonite R., Gately M. K. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992 May 15;148(10):3125–3132. [PubMed] [Google Scholar]
- Fitch F. W., McKisic M. D., Lancki D. W., Gajewski T. F. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29–48. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- Gaulton G. N., Bangs J., Maddock S., Springer T., Eardley D. D., Strom T. B. Characterization of a monoclonal rat anti-mouse interleukin 2 (IL-2) receptor antibody and its use in the biochemical characterization of the murine IL-2 receptor. Clin Immunol Immunopathol. 1985 Jul;36(1):18–29. doi: 10.1016/0090-1229(85)90035-2. [DOI] [PubMed] [Google Scholar]
- Gavett S. H., O'Hearn D. J., Li X., Huang S. K., Finkelman F. D., Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995 Nov 1;182(5):1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrzak J. A., Brunda M. J. Interleukin-12. Biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995 Jun;72(6):619–637. [PubMed] [Google Scholar]
- Kubin M., Kamoun M., Trinchieri G. Interleukin 12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994 Jul 1;180(1):211–222. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J. P., Waldburger K. E., Goldman S. J. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995 Jan 1;181(1):381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo S., Toyo-oka K., Oh-hora M., Tai X. G., Iwata H., Takenaka H., Yamada S., Ono S., Hamaoka T., Kobayashi M. IL-12 produced by antigen-presenting cells induces IL-2-independent proliferation of T helper cell clones. J Immunol. 1996 Mar 1;156(5):1748–1755. [PubMed] [Google Scholar]
- McIntyre K. W., Shuster D. J., Gillooly K. M., Warrier R. R., Connaughton S. E., Hall L. B., Arp L. H., Gately M. K., Magram J. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur J Immunol. 1996 Dec;26(12):2933–2938. doi: 10.1002/eji.1830261219. [DOI] [PubMed] [Google Scholar]
- McKnight A. J., Zimmer G. J., Fogelman I., Wolf S. F., Abbas A. K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994 Mar 1;152(5):2172–2179. [PubMed] [Google Scholar]
- McMurray R. W., Sharp G. C., Braley-Mullen H. Intrathyroidal cell phenotype in murine lymphocytic and granulomatous experimental autoimmune thyroiditis. Autoimmunity. 1994;18(2):93–102. doi: 10.3109/08916939409007982. [DOI] [PubMed] [Google Scholar]
- Murphy E. E., Terres G., Macatonia S. E., Hsieh C. S., Mattson J., Lanier L., Wysocka M., Trinchieri G., Murphy K., O'Garra A. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994 Jul 1;180(1):223–231. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman E., Lass J. H., Bardenstein D. S., Diaconu E., Hazlett F. E., Jr, Albright J., Higgins A. W., Kazura J. W. IL-12 exacerbates helminth-mediated corneal pathology by augmenting inflammatory cell recruitment and chemokine expression. J Immunol. 1997 Jan 15;158(2):827–833. [PubMed] [Google Scholar]
- Schmitt E., Hoehn P., Germann T., Rüde E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994 Feb;24(2):343–347. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Gazzinelli R., Sher A., Paul W. E. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seder R. A., Kelsall B. L., Jankovic D. Differential roles for IL-12 in the maintenance of immune responses in infectious versus autoimmune disease. J Immunol. 1996 Oct 1;157(7):2745–2748. [PubMed] [Google Scholar]
- Stull S. J., Kyriakos M., Sharp G. C., Braley-Mullen H. Prevention and reversal of experimental autoimmune thyroiditis (EAT) in mice by administration of anti-L3T4 monoclonal antibody at different stages of disease development. Cell Immunol. 1988 Nov;117(1):188–198. doi: 10.1016/0008-8749(88)90087-1. [DOI] [PubMed] [Google Scholar]
- Stull S. J., Sharp G. C., Kyriakos M., Bickel J. T., Braley-Mullen H. Induction of granulomatous experimental autoimmune thyroiditis in mice with in vitro activated effector T cells and anti-IFN-gamma antibody. J Immunol. 1992 Sep 15;149(6):2219–2226. [PubMed] [Google Scholar]
- Tang H., Braley-Mullen H. Intravenous administration of deaggregated mouse thyroglobulin suppresses induction of experimental autoimmune thyroiditis and expression of both Th1 and Th2 cytokines. Int Immunol. 1997 May;9(5):679–687. doi: 10.1093/intimm/9.5.679. [DOI] [PubMed] [Google Scholar]
- Tang H., Sharp G. C., Peterson K. E., Braley-Mullen H. Induction of granulomatous experimental autoimmune thyroiditis in IL-4 gene-disrupted mice. J Immunol. 1998 Jan 1;160(1):155–162. [PubMed] [Google Scholar]
- Trembleau S., Penna G., Bosi E., Mortara A., Gately M. K., Adorini L. Interleukin 12 administration induces T helper type 1 cells and accelerates autoimmune diabetes in NOD mice. J Exp Med. 1995 Feb 1;181(2):817–821. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- Waldburger K. E., Hastings R. C., Schaub R. G., Goldman S. J., Leonard J. P. Adoptive transfer of experimental allergic encephalomyelitis after in vitro treatment with recombinant murine interleukin-12. Preferential expansion of interferon-gamma-producing cells and increased expression of macrophage-associated inducible nitric oxide synthase as immunomodulatory mechanisms. Am J Pathol. 1996 Feb;148(2):375–382. [PMC free article] [PubMed] [Google Scholar]
- Wang Z. E., Zheng S., Corry D. B., Dalton D. K., Seder R. A., Reiner S. L., Locksley R. M. Interferon gamma-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12932–12936. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A., Jankovic D., Hieny S., Cheever A. W., Sher A. IL-12 enhances vaccine-induced immunity to Schistosoma mansoni in mice and decreases T helper 2 cytokine expression, IgE production, and tissue eosinophilia. J Immunol. 1995 May 1;154(9):4701–4709. [PubMed] [Google Scholar]
- Wynn T. A., Jankovic D., Hieny S., Zioncheck K., Jardieu P., Cheever A. W., Sher A. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. J Immunol. 1995 Apr 15;154(8):3999–4009. [PubMed] [Google Scholar]
- Yokoi H., Kato K., Kezuka T., Sakai J., Usui M., Yagita H., Okumura K. Prevention of experimental autoimmune uveoretinitis by monoclonal antibody to interleukin-12. Eur J Immunol. 1997 Mar;27(3):641–646. doi: 10.1002/eji.1830270310. [DOI] [PubMed] [Google Scholar]