Abstract
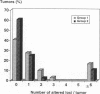
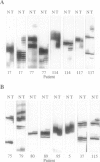
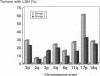
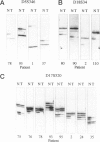
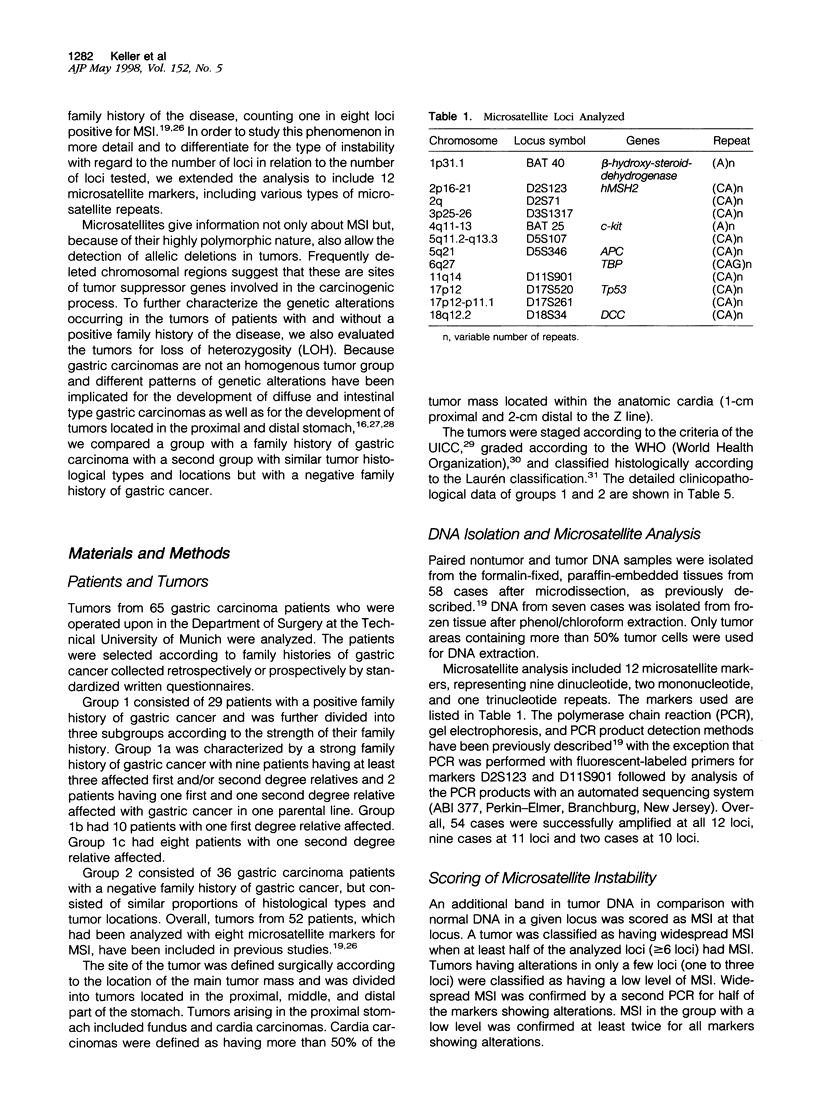
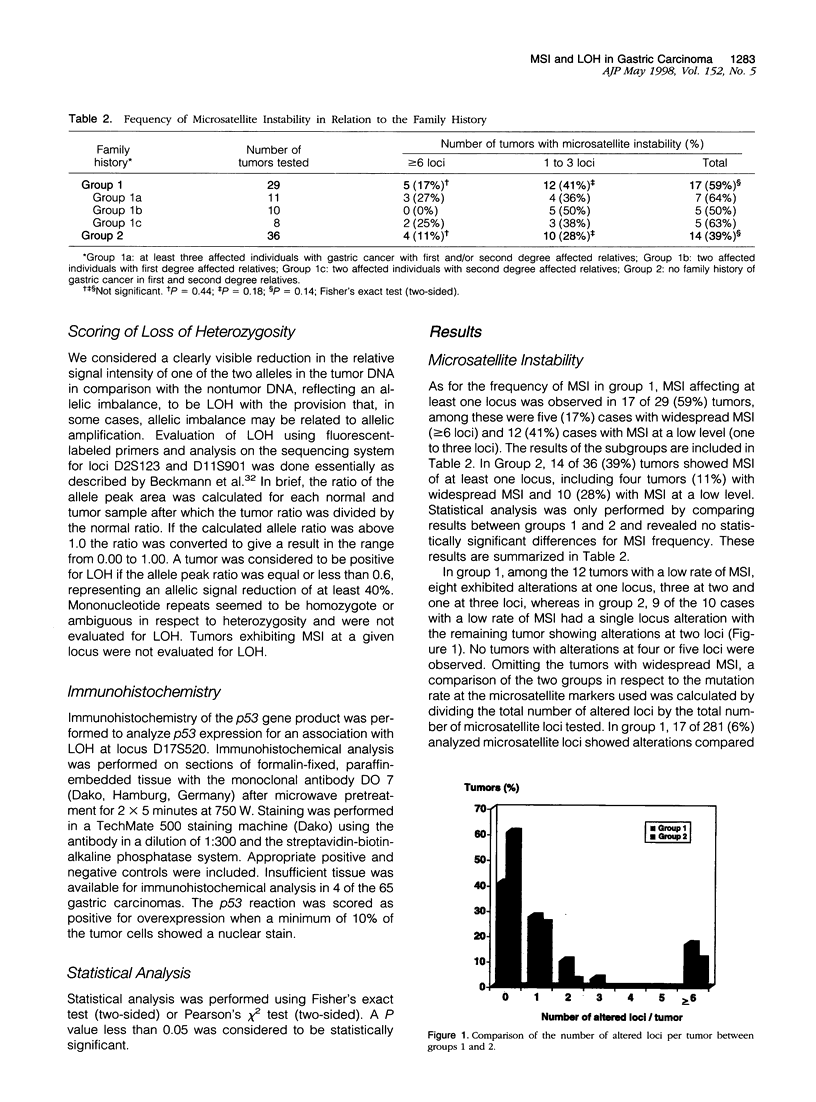
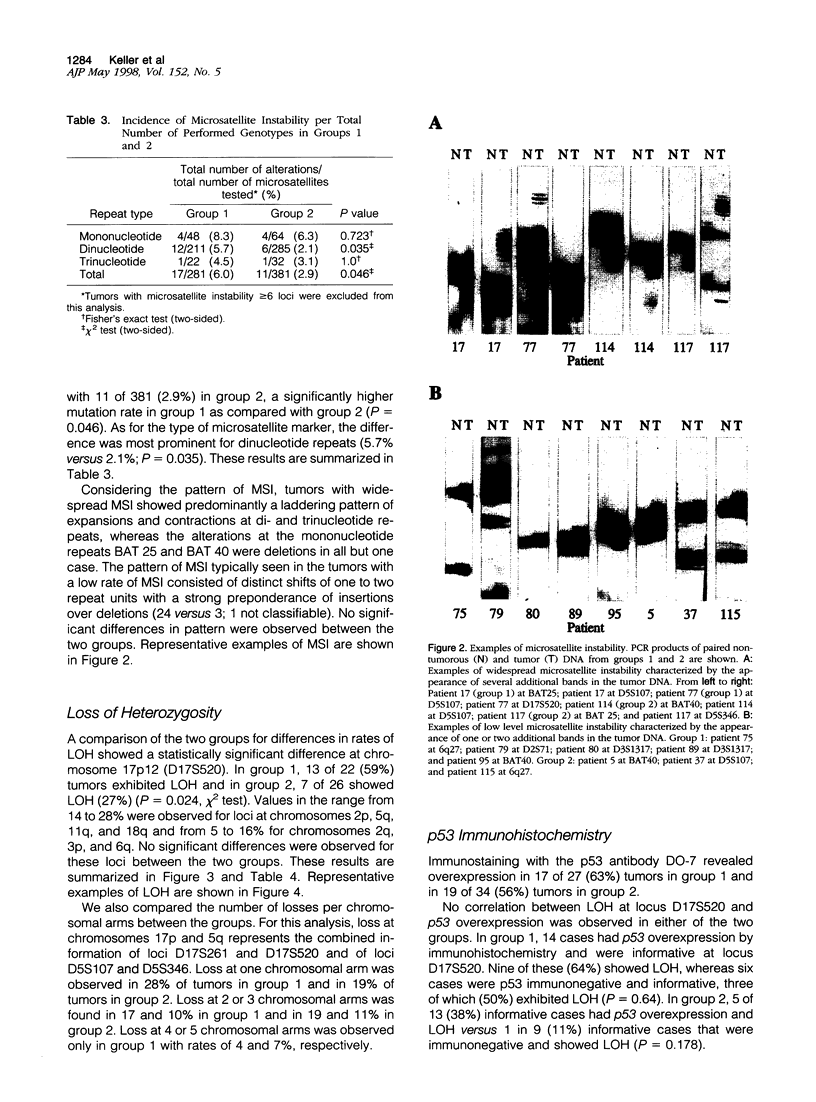
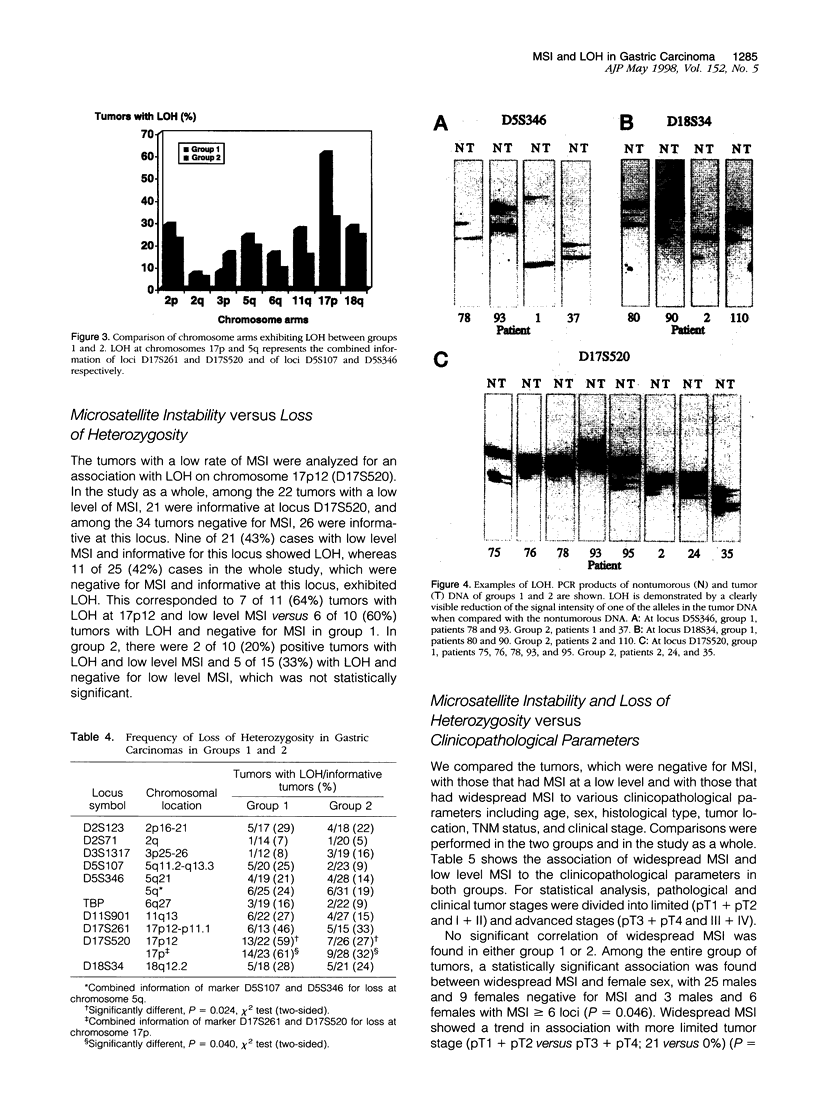
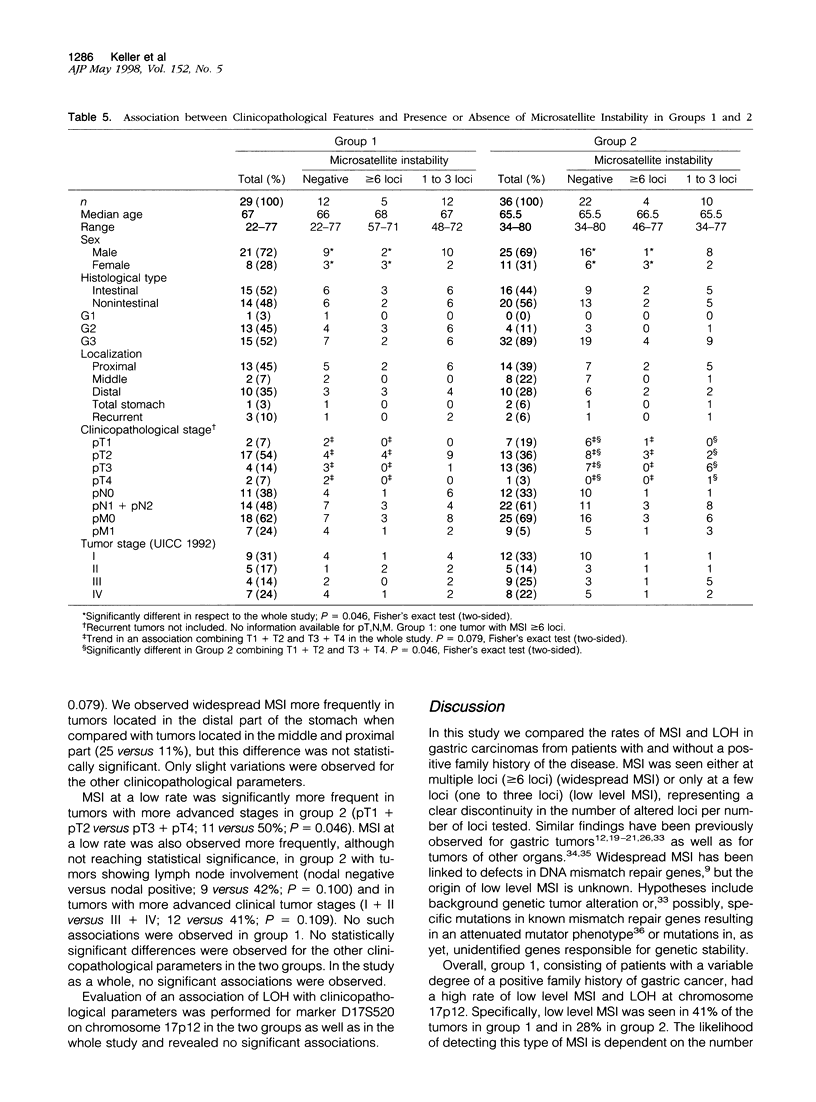
We compared 29 gastric carcinomas from patients with a variably strong family history for gastric cancer (group 1) with 36 gastric carcinomas from patients without a family history of this disease (group 2) for microsatellite instability (MSI) and loss of heterozygosity (LOH) with 12 microsatellite markers. Both study groups had similar proportions of histological types and tumor locations. Widespread MSI (alterations at > or = 6 loci) was seen in 5 of 29 (17%) of the tumors belonging to group 1 and in 4 of 36 (11%) group 2 tumors. MSI at a low level (alterations at 1 to 3 loci) was observed in 12 of 29 (41%) of tumors in group 1 and in 10 of 36 (28%) of tumors in group 2, differences that were not statistically significant. A significant difference with respect to low level MSI was observed between the two groups when considering the overall mutation rate of microsatellites. Seventeen of 281 (6%) analyzed microsatellite loci showed alterations in group 1 and 11 of 381 (2.9%) in group 2 (P = 0.046). Comparison of both types of MSI to the clinicopathological parameters in both groups revealed a significant association of low level MSI with advanced tumor stages (P = 0.046) in the group 2, whereas no such association was observed in group 1. In respect to LOH, a significant difference between the two groups was observed at chromosome 17p12, as 13 of 22 (59%) informative cases of group 1 showed LOH in comparison with 7 of 26 (27%) (P = 0.024) in group 2. No correlation of LOH at chromosome 17p12 to the pathological or clinical data was observed either in the two groups or in the study as a whole. Our data show that gastric carcinomas of patients with a positive family history of gastric cancer in group 1 are characterized by a higher mutation rate in respect to low level MSI, particularly at dinucleotide repeats, and by a higher frequency of LOH at chromosome 17p12, indicating that different genetic pathways are involved in the pathogenesis of gastric carcinomas arising in patients with and without a familial background of this disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Amos W., Sawcer S. J., Feakes R. W., Rubinsztein D. C. Microsatellites show mutational bias and heterozygote instability. Nat Genet. 1996 Aug;13(4):390–391. doi: 10.1038/ng0896-390. [DOI] [PubMed] [Google Scholar]
- Baas I. O., Mulder J. W., Offerhaus G. J., Vogelstein B., Hamilton S. R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994 Jan;172(1):5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Becker K. F., Atkinson M. J., Reich U., Becker I., Nekarda H., Siewert J. R., Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994 Jul 15;54(14):3845–3852. [PubMed] [Google Scholar]
- Beckmann M. W., Picard F., An H. X., van Roeyen C. R., Dominik S. I., Mosny D. S., Schnürch H. G., Bender H. G., Niederacher D. Clinical impact of detection of loss of heterozygosity of BRCA1 and BRCA2 markers in sporadic breast cancer. Br J Cancer. 1996 May;73(10):1220–1226. doi: 10.1038/bjc.1996.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T., Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994 Jan-Feb;44(1):7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- Buonsanti G., Calistri D., Padovan L., Luinetti O., Fiocca R., Solcia E., Ranzani G. N. Microsatellite instability in intestinal- and diffuse-type gastric carcinoma. J Pathol. 1997 Jun;182(2):167–173. doi: 10.1002/(SICI)1096-9896(199706)182:2<167::AID-PATH830>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Chong J. M., Fukayama M., Hayashi Y., Takizawa T., Koike M., Konishi M., Kikuchi-Yanoshita R., Miyaki M. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994 Sep 1;54(17):4595–4597. [PubMed] [Google Scholar]
- Christiansen J. The intestinal phase of gastric acid secretion in man. Scand J Gastroenterol. 1978;13(1):1–2. doi: 10.3109/00365527809179799. [DOI] [PubMed] [Google Scholar]
- Chung Y. J., Song J. M., Lee J. Y., Jung Y. T., Seo E. J., Choi S. W., Rhyu M. G. Microsatellite instability-associated mutations associate preferentially with the intestinal type of primary gastric carcinomas in a high-risk population. Cancer Res. 1996 Oct 15;56(20):4662–4665. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992 Dec 15;52(24):6735–6740. [PubMed] [Google Scholar]
- Eshleman J. R., Markowitz S. D. Mismatch repair defects in human carcinogenesis. Hum Mol Genet. 1996;5(Spec No):1489–1494. doi: 10.1093/hmg/5.supplement_1.1489. [DOI] [PubMed] [Google Scholar]
- Fey M. F., Hesketh C., Wainscoat J. S., Gendler S., Thein S. L. Clonal allele loss in gastrointestinal cancers. Br J Cancer. 1989 May;59(5):750–754. doi: 10.1038/bjc.1989.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbert H. E., Müller W., Schneiders A., Meier S., Hommel G. The relationship of p53 expression to the prognosis of 418 patients with gastric carcinoma. Cancer. 1995 Sep 1;76(5):720–726. doi: 10.1002/1097-0142(19950901)76:5<720::aid-cncr2820760503>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Glebov O. K., McKenzie K. E., White C. A., Sukumar S. Frequent p53 gene mutations and novel alleles in familial breast cancer. Cancer Res. 1994 Jul 15;54(14):3703–3709. [PubMed] [Google Scholar]
- Gleeson C. M., Sloan J. M., McGuigan J. A., Ritchie A. J., Weber J. L., Russell S. E. Widespread microsatellite instability occurs infrequently in adenocarcinoma of the gastric cardia. Oncogene. 1996 Apr 18;12(8):1653–1662. [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Honchel R., Halling K. C., Thibodeau S. N. Genomic instability in neoplasia. Semin Cell Biol. 1995 Feb;6(1):45–52. doi: 10.1016/1043-4682(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Keller G., Grimm V., Vogelsang H., Bischoff P., Mueller J., Siewert J. R., Höfler H. Analysis for microsatellite instability and mutations of the DNA mismatch repair gene hMLH1 in familial gastric cancer. Int J Cancer. 1996 Nov 27;68(5):571–576. doi: 10.1002/(SICI)1097-0215(19961127)68:5<571::AID-IJC3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Keller G., Rotter M., Vogelsang H., Bischoff P., Becker K. F., Mueller J., Brauch H., Siewert J. R., Höfler H. Microsatellite instability in adenocarcinomas of the upper gastrointestinal tract. Relation to clinicopathological data and family history. Am J Pathol. 1995 Sep;147(3):593–600. [PMC free article] [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Negri E., Franceschi S., Gentile A. Family history and the risk of stomach and colorectal cancer. Cancer. 1992 Jul 1;70(1):50–55. doi: 10.1002/1097-0142(19920701)70:1<50::aid-cncr2820700109>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lim B. H., Soong R., Grieu F., Robbins P. D., House A. K., Iacopetta B. J. p53 accumulation and mutation are prognostic indicators of poor survival in human gastric carcinoma. Int J Cancer. 1996 Jun 21;69(3):200–204. doi: 10.1002/(SICI)1097-0215(19960621)69:3<200::AID-IJC9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Lin J. T., Wu M. S., Shun C. T., Lee W. J., Wang J. T., Wang T. H., Sheu J. C. Microsatellite instability in gastric carcinoma with special references to histopathology and cancer stages. Eur J Cancer. 1995 Oct;31A(11):1879–1882. doi: 10.1016/0959-8049(95)00349-n. [DOI] [PubMed] [Google Scholar]
- Lindstedt B. A., Ryberg D., Haugen A. Rare alleles at different VNTR loci among lung-cancer patients with microsatellite instability in tumours. Int J Cancer. 1997 Feb 7;70(4):412–415. doi: 10.1002/(sici)1097-0215(19970207)70:4<412::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Mironov N. M., Aguelon M. A., Potapova G. I., Omori Y., Gorbunov O. V., Klimenkov A. A., Yamasaki H. Alterations of (CA)n DNA repeats and tumor suppressor genes in human gastric cancer. Cancer Res. 1994 Jan 1;54(1):41–44. [PubMed] [Google Scholar]
- Ottini L., Palli D., Falchetti M., D'Amico C., Amorosi A., Saieva C., Calzolari A., Cimoli F., Tatarelli C., De Marchis L. Microsatellite instability in gastric cancer is associated with tumor location and family history in a high-risk population from Tuscany. Cancer Res. 1997 Oct 15;57(20):4523–4529. [PubMed] [Google Scholar]
- Palli D., Galli M., Caporaso N. E., Cipriani F., Decarli A., Saieva C., Fraumeni J. F., Jr, Buiatti E. Family history and risk of stomach cancer in Italy. Cancer Epidemiol Biomarkers Prev. 1994 Jan-Feb;3(1):15–18. [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Liu B., Parsons R., Lengauer C., Palombo F., D'Arrigo A., Markowitz S., Willson J. K., Kinzler K. W. Mutations of GTBP in genetically unstable cells. Science. 1995 Jun 30;268(5219):1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- Peltomäki P., Lothe R. A., Aaltonen L. A., Pylkkänen L., Nyström-Lahti M., Seruca R., David L., Holm R., Ryberg D., Haugen A. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993 Dec 15;53(24):5853–5855. [PubMed] [Google Scholar]
- Peltomäki P., Vasen H. F. Mutations predisposing to hereditary nonpolyposis colorectal cancer: database and results of a collaborative study. The International Collaborative Group on Hereditary Nonpolyposis Colorectal Cancer. Gastroenterology. 1997 Oct;113(4):1146–1158. doi: 10.1053/gast.1997.v113.pm9322509. [DOI] [PubMed] [Google Scholar]
- Ranzani G. N., Renault B., Pellegata N. S., Fattorini P., Magni E., Bacci F., Amadori D. Loss of heterozygosity and K-ras gene mutations in gastric cancer. Hum Genet. 1993 Oct 1;92(3):244–249. doi: 10.1007/BF00244466. [DOI] [PubMed] [Google Scholar]
- Renault B., Calistri D., Buonsanti G., Nanni O., Amadori D., Ranzani G. N. Microsatellite instability and mutations of p53 and TGF-beta RII genes in gastric cancer. Hum Genet. 1996 Nov;98(5):601–607. doi: 10.1007/s004390050267. [DOI] [PubMed] [Google Scholar]
- Rhyu M. G., Park W. S., Meltzer S. J. Microsatellite instability occurs frequently in human gastric carcinoma. Oncogene. 1994 Jan;9(1):29–32. [PubMed] [Google Scholar]
- Ryberg D., Lindstedt B. A., Zienolddiny S., Haugen A. A hereditary genetic marker closely associated with microsatellite instability in lung cancer. Cancer Res. 1995 Sep 15;55(18):3996–3999. [PubMed] [Google Scholar]
- Sano T., Tsujino T., Yoshida K., Nakayama H., Haruma K., Ito H., Nakamura Y., Kajiyama G., Tahara E. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res. 1991 Jun 1;51(11):2926–2931. [PubMed] [Google Scholar]
- Semba S., Yokozaki H., Yamamoto S., Yasui W., Tahara E. Microsatellite instability in precancerous lesions and adenocarcinomas of the stomach. Cancer. 1996 Apr 15;77(8 Suppl):1620–1627. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1620::AID-CNCR30>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shinmura K., Yin W., Isogaki J., Saitoh K., Kanazawa K., Koda K., Yokota J., Kino I., Arai T., Sugimura H. Stage-dependent evaluation of microsatellite instability in gastric carcinoma with familial clustering. Cancer Epidemiol Biomarkers Prev. 1997 Sep;6(9):693–697. [PubMed] [Google Scholar]
- Simpson A. J. The natural somatic mutation frequency and human carcinogenesis. Adv Cancer Res. 1997;71:209–240. doi: 10.1016/s0065-230x(08)60100-1. [DOI] [PubMed] [Google Scholar]
- Soong R., Robbins P. D., Dix B. R., Grieu F., Lim B., Knowles S., Williams K. E., Turbett G. R., House A. K., Iacopetta B. J. Concordance between p53 protein overexpression and gene mutation in a large series of common human carcinomas. Hum Pathol. 1996 Oct;27(10):1050–1055. doi: 10.1016/s0046-8177(96)90282-8. [DOI] [PubMed] [Google Scholar]
- Strickler J. G., Zheng J., Shu Q., Burgart L. J., Alberts S. R., Shibata D. p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res. 1994 Sep 1;54(17):4750–4755. [PubMed] [Google Scholar]
- Tahara E. Genetic alterations in human gastrointestinal cancers. The application to molecular diagnosis. Cancer. 1995 Mar 15;75(6 Suppl):1410–1417. doi: 10.1002/1097-0142(19950315)75:6+<1410::aid-cncr2820751504>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Tamura G., Sakata K., Maesawa C., Suzuki Y., Terashima M., Satoh K., Sekiyama S., Suzuki A., Eda Y., Satodate R. Microsatellite alterations in adenoma and differentiated adenocarcinoma of the stomach. Cancer Res. 1995 May 1;55(9):1933–1936. [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Zanghieri G., Di Gregorio C., Sacchetti C., Fante R., Sassatelli R., Cannizzo G., Carriero A., Ponz de Leon M. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer. 1990 Nov 1;66(9):2047–2051. doi: 10.1002/1097-0142(19901101)66:9<2047::aid-cncr2820660934>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- dos Santos N. R., Seruca R., Constância M., Seixas M., Sobrinho-Simões M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996 Jan;110(1):38–44. doi: 10.1053/gast.1996.v110.pm8536886. [DOI] [PubMed] [Google Scholar]