Abstract
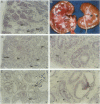
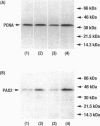
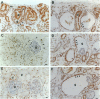
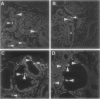
Renal malformations account for most children with chronic renal failure and are often associated with urinary tract anatomical obstruction. We examined cellular and molecular events after experimental urinary flow impairment in fetal sheep. Ovine gestation lasts 144 to 150 days with the metanephros appearing at 27 to 30 days. We generated complete unilateral ureteric anatomical obstruction at 90 days when a few layers of glomeruli had formed. After 10 days, we recorded ureteric and pelvic dilatation with renal parenchymal weight greater than contralateral organs or those from unoperated fetuses. The nephrogenic cortex was replaced by disorganized cells separated by edema and prominent vascular spaces. Cortical histology was dominated by cysts associated with malformed glomerular tufts. Cystic epithelia expressed PAX2, a growth-stimulating transcription factor down-regulated during normal maturation, and proliferating cell nuclear antigen, a surrogate marker of cycling cells. Detection of apoptosis using propidium iodide and in situ end labeling showed a significant increase of the point prevalence of death in the obstructed cortex. Hence, PAX2 and proliferating cell nuclear antigen expression as well as death were deregulated, as we previously reported in human kidney malformations. Medullary collecting ducts and loops of Henle were also disrupted, correlating with impaired urinary dilution and sodium reabsorption. Therefore, complex aberrations of morphogenesis, gene expression, cell turnover, and urine composition occur relatively early after experimental impairment of fetal urinary flow.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck A. D. The effect of intra-uterine urinary obstruction upon the development of the fetal kidney. J Urol. 1971 Jun;105(6):784–789. doi: 10.1016/s0022-5347(17)61629-x. [DOI] [PubMed] [Google Scholar]
- Berman D. J., Maizels M. The role of urinary obstruction in the genesis of renal dysplasia. A model in the chick embryo. J Urol. 1982 Nov;128(5):1091–1096. doi: 10.1016/s0022-5347(17)53351-0. [DOI] [PubMed] [Google Scholar]
- Bernstein J. Glomerulocystic kidney disease--nosological considerations. Pediatr Nephrol. 1993 Aug;7(4):464–470. doi: 10.1007/BF00857576. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Carr M. C., Schlussel R. N., Peters C. A., Uchida T., Mandell J., Freeman M. R. Expression of cell growth regulated genes in the fetal kidney: relevance to in utero obstruction. J Urol. 1995 Jul;154(1):242–246. [PubMed] [Google Scholar]
- Chevalier R. L. Growth factors and apoptosis in neonatal ureteral obstruction. J Am Soc Nephrol. 1996 Aug;7(8):1098–1105. doi: 10.1681/ASN.V781098. [DOI] [PubMed] [Google Scholar]
- Chevalier R. L., Thornhill B. A., Gomez R. A. EDRF modulates renal hemodynamics during unilateral ureteral obstruction in the rat. Kidney Int. 1992 Aug;42(2):400–406. doi: 10.1038/ki.1992.301. [DOI] [PubMed] [Google Scholar]
- Coles H. S., Burne J. F., Raff M. C. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993 Jul;118(3):777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Coplen D. E. Prenatal intervention for hydronephrosis. J Urol. 1997 Jun;157(6):2270–2277. [PubMed] [Google Scholar]
- Dressler G. R., Deutsch U., Chowdhury K., Nornes H. O., Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990 Aug;109(4):787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dressler G. R., Douglass E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1179–1183. doi: 10.1073/pnas.89.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G. R., Wilkinson J. E., Rothenpieler U. W., Patterson L. T., Williams-Simons L., Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993 Mar 4;362(6415):65–67. doi: 10.1038/362065a0. [DOI] [PubMed] [Google Scholar]
- Eccles M. R., Wallis L. J., Fidler A. E., Spurr N. K., Goodfellow P. J., Reeve A. E. Expression of the PAX2 gene in human fetal kidney and Wilms' tumor. Cell Growth Differ. 1992 May;3(5):279–289. [PubMed] [Google Scholar]
- Feather S. A., Winyard P. J., Dodd S., Woolf A. S. Oral-facial-digital syndrome type 1 is another dominant polycystic kidney disease: clinical, radiological and histopathological features of a new kindred. Nephrol Dial Transplant. 1997 Jul;12(7):1354–1361. doi: 10.1093/ndt/12.7.1354. [DOI] [PubMed] [Google Scholar]
- Feather S. A., Woolf A. S., Donnai D., Malcolm S., Winter R. M. The oral-facial-digital syndrome type 1 (OFD1), a cause of polycystic kidney disease and associated malformations, maps to Xp22.2-Xp22.3. Hum Mol Genet. 1997 Jul;6(7):1163–1167. doi: 10.1093/hmg/6.7.1163. [DOI] [PubMed] [Google Scholar]
- Foxall P. J., Price R. G., Jones J. K., Neild G. H., Thompson F. D., Nicholson J. K. High resolution proton magnetic resonance spectroscopy of cyst fluids from patients with polycystic kidney disease. Biochim Biophys Acta. 1992 Apr 14;1138(4):305–314. doi: 10.1016/0925-4439(92)90008-b. [DOI] [PubMed] [Google Scholar]
- Gage J. C., Sulik K. K. Pathogenesis of ethanol-induced hydronephrosis and hydroureter as demonstrated following in vivo exposure of mouse embryos. Teratology. 1991 Sep;44(3):299–312. doi: 10.1002/tera.1420440307. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T., Lelievre-Pegorier M., Malienou R., Meulemans A., Merlet-Benichou C. Effects of prenatal and postnatal exposure to gentamicin on renal differentiation in the rat. Toxicology. 1987 Mar;43(3):301–313. doi: 10.1016/0300-483x(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Glazebrook K. N., McGrath F. P., Steele B. T. Prenatal compensatory renal growth: documentation with US. Radiology. 1993 Dec;189(3):733–735. doi: 10.1148/radiology.189.3.8234697. [DOI] [PubMed] [Google Scholar]
- Gnarra J. R., Dressler G. R. Expression of Pax-2 in human renal cell carcinoma and growth inhibition by antisense oligonucleotides. Cancer Res. 1995 Sep 15;55(18):4092–4098. [PubMed] [Google Scholar]
- Granata C., Wang Y., Puri P., Tanaka K., O'Briain D. S. Decreased bcl-2 expression in segmental renal dysplasia suggests a role in its morphogenesis. Br J Urol. 1997 Jul;80(1):140–144. doi: 10.1046/j.1464-410x.1997.00235.x. [DOI] [PubMed] [Google Scholar]
- Grantham J. J. 1992 Homer Smith Award. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol. 1993 Jun;3(12):1841–1857. doi: 10.1681/ASN.V3121841. [DOI] [PubMed] [Google Scholar]
- Kaneto H., Morrissey J., Klahr S. Increased expression of TGF-beta 1 mRNA in the obstructed kidney of rats with unilateral ureteral ligation. Kidney Int. 1993 Aug;44(2):313–321. doi: 10.1038/ki.1993.246. [DOI] [PubMed] [Google Scholar]
- Koseki C., Herzlinger D., al-Awqati Q. Apoptosis in metanephric development. J Cell Biol. 1992 Dec;119(5):1327–1333. doi: 10.1083/jcb.119.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughna S., Hardman P., Landels E., Jussila L., Alitalo K., Woolf A. S. A molecular and genetic analysis of renalglomerular capillary development. Angiogenesis. 1997;1(1):84–101. doi: 10.1023/A:1018357116559. [DOI] [PubMed] [Google Scholar]
- Mandell J., Peters C. A., Estroff J. A., Allred E. N., Benacerraf B. R. Human fetal compensatory renal growth. J Urol. 1993 Aug;150(2 Pt 2):790–792. doi: 10.1016/s0022-5347(17)35614-8. [DOI] [PubMed] [Google Scholar]
- Matsell D. G., Bennett T., Armstrong R. A., Goodyer P., Goodyer C., Han V. K. Insulin-like growth factor (IGF) and IGF binding protein gene expression in multicystic renal dysplasia. J Am Soc Nephrol. 1997 Jan;8(1):85–94. doi: 10.1681/ASN.V8185. [DOI] [PubMed] [Google Scholar]
- Maulbecker C. C., Gruss P. The oncogenic potential of Pax genes. EMBO J. 1993 Jun;12(6):2361–2367. doi: 10.1002/j.1460-2075.1993.tb05890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery P. T., Stablein D. M., Arbus G., Tejani A. Renal transplantation in children. A report of the North American Pediatric Renal Transplant Cooperative Study. N Engl J Med. 1992 Jun 25;326(26):1727–1732. doi: 10.1056/NEJM199206253262602. [DOI] [PubMed] [Google Scholar]
- Mesrobian H. G., Rushton H. G., Bulas D. Unilateral renal agenesis may result from in utero regression of multicystic renal dysplasia. J Urol. 1993 Aug;150(2 Pt 2):793–794. doi: 10.1016/s0022-5347(17)35615-x. [DOI] [PubMed] [Google Scholar]
- Nadasdy T., Laszik Z., Lajoie G., Blick K. E., Wheeler D. E., Silva F. G. Proliferative activity of cyst epithelium in human renal cystic diseases. J Am Soc Nephrol. 1995 Jan;5(7):1462–1468. doi: 10.1681/ASN.V571462. [DOI] [PubMed] [Google Scholar]
- Peters C. A., Carr M. C., Lais A., Retik A. B., Mandell J. The response of the fetal kidney to obstruction. J Urol. 1992 Aug;148(2 Pt 2):503–509. doi: 10.1016/s0022-5347(17)36640-5. [DOI] [PubMed] [Google Scholar]
- Peters C. A., Gaertner R. C., Carr M. C., Mandell J. Fetal compensatory renal growth due to unilateral ureteral obstruction. J Urol. 1993 Aug;150(2 Pt 2):597–600. doi: 10.1016/s0022-5347(17)35559-3. [DOI] [PubMed] [Google Scholar]
- Peters C. A. Obstruction of the fetal urinary tract. J Am Soc Nephrol. 1997 Apr;8(4):653–663. doi: 10.1681/ASN.V84653. [DOI] [PubMed] [Google Scholar]
- Peters C. A. Urinary tract obstruction in children. J Urol. 1995 Nov;154(5):1874–1884. doi: 10.1016/s0022-5347(01)66815-0. [DOI] [PubMed] [Google Scholar]
- Phelps D. E., Dressler G. R. Identification of novel Pax-2 binding sites by chromatin precipitation. J Biol Chem. 1996 Apr 5;271(14):7978–7985. doi: 10.1074/jbc.271.14.7978. [DOI] [PubMed] [Google Scholar]
- Qian F., Watnick T. J., Onuchic L. F., Germino G. G. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996 Dec 13;87(6):979–987. doi: 10.1016/s0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- Rogers S. A., Ryan G., Purchio A. F., Hammerman M. R. Metanephric transforming growth factor-beta 1 regulates nephrogenesis in vitro. Am J Physiol. 1993 Jun;264(6 Pt 2):F996–1002. doi: 10.1152/ajprenal.1993.264.6.F996. [DOI] [PubMed] [Google Scholar]
- Rothenpieler U. W., Dressler G. R. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993 Nov;119(3):711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Sanyanusin P., Schimmenti L. A., McNoe L. A., Ward T. A., Pierpont M. E., Sullivan M. J., Dobyns W. B., Eccles M. R. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet. 1995 Apr;9(4):358–364. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- Steinhardt G. F., Liapis H., Phillips B., Vogler G., Nag M., Yoon K. W. Insulin-like growth factor improves renal architecture of fetal kidneys with complete ureteral obstruction. J Urol. 1995 Aug;154(2 Pt 2):690–693. doi: 10.1097/00005392-199508000-00093. [DOI] [PubMed] [Google Scholar]
- Stuart E. T., Haffner R., Oren M., Gruss P. Loss of p53 function through PAX-mediated transcriptional repression. EMBO J. 1995 Nov 15;14(22):5638–5645. doi: 10.1002/j.1460-2075.1995.tb00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagho E. A. Surgically induced partial urinary obstruction in the fetal lamb. 3. Ureteral obstruction. Invest Urol. 1972 Jul;10(1):35–52. [PubMed] [Google Scholar]
- Tanner G. A., McQuillan P. F., Maxwell M. R., Keck J. K., McAteer J. A. An in vitro test of the cell stretch-proliferation hypothesis of renal cyst enlargement. J Am Soc Nephrol. 1995 Oct;6(4):1230–1241. doi: 10.1681/ASN.V641230. [DOI] [PubMed] [Google Scholar]
- Torres M., Gómez-Pardo E., Dressler G. R., Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995 Dec;121(12):4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Truong L. D., Petrusevska G., Yang G., Gurpinar T., Shappell S., Lechago J., Rouse D., Suki W. N. Cell apoptosis and proliferation in experimental chronic obstructive uropathy. Kidney Int. 1996 Jul;50(1):200–207. doi: 10.1038/ki.1996.303. [DOI] [PubMed] [Google Scholar]
- Veis D. J., Sorenson C. M., Shutter J. R., Korsmeyer S. J. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993 Oct 22;75(2):229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Wintour E. M., Alcorn D., Butkus A., Congiu M., Earnest L., Pompolo S., Potocnik S. J. Ontogeny of hormonal and excretory function of the meso- and metanephros in the ovine fetus. Kidney Int. 1996 Nov;50(5):1624–1633. doi: 10.1038/ki.1996.478. [DOI] [PubMed] [Google Scholar]
- Winyard P. J., Bao Q., Hughes R. C., Woolf A. S. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol. 1997 Nov;8(11):1647–1657. doi: 10.1681/ASN.V8111647. [DOI] [PubMed] [Google Scholar]
- Winyard P. J., Nauta J., Lirenman D. S., Hardman P., Sams V. R., Risdon R. A., Woolf A. S. Deregulation of cell survival in cystic and dysplastic renal development. Kidney Int. 1996 Jan;49(1):135–146. doi: 10.1038/ki.1996.18. [DOI] [PubMed] [Google Scholar]
- Winyard P. J., Risdon R. A., Sams V. R., Dressler G. R., Woolf A. S. The PAX2 tanscription factor is expressed in cystic and hyperproliferative dysplastic epithelia in human kidney malformations. J Clin Invest. 1996 Jul 15;98(2):451–459. doi: 10.1172/JCI118811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo D. D., Miao S. Y., Pelayo J. C., Woolf A. S. Taxol inhibits progression of congenital polycystic kidney disease. Nature. 1994 Apr 21;368(6473):750–753. doi: 10.1038/368750a0. [DOI] [PubMed] [Google Scholar]
- Woo D. Apoptosis and loss of renal tissue in polycystic kidney diseases. N Engl J Med. 1995 Jul 6;333(1):18–25. doi: 10.1056/NEJM199507063330104. [DOI] [PubMed] [Google Scholar]
- Woolf A. S., Cale C. M. Roles of growth factors in renal development. Curr Opin Nephrol Hypertens. 1997 Jan;6(1):10–14. doi: 10.1097/00041552-199701000-00003. [DOI] [PubMed] [Google Scholar]
- Woolf A. S. Multiple causes of human kidney malformations. Arch Dis Child. 1997 Dec;77(6):471–473. doi: 10.1136/adc.77.6.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Horiuchi M., Dzau V. J. Angiotensin II type 2 receptor mediates programmed cell death. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]