Abstract
Proteasome is a major cellular organelle responsible for the regulated turn-over of both normal and misfolded proteins. Recent reports from our laboratory have implicated lowered proteasomal chymotryptic activity to be responsible for decreased induction of the transcription factor NFκB in T lymphocytes during aging. In this study, we have further analyzed the basis for this decline in proteasomal function, by focusing on the role of oxidative stress. Upon exposure to the pro-oxidant BSO, both ATP-stimulatable 26S, as well as ATP-independent 20S proteasomal catalytic activity could be down-regulated in T cells from young donors, mimicking the decline observed in T cells from the elderly. Loss in these catalytic activities, following exposure to pro-oxidant stimulus also resulted in a decline in both activation-induced proliferation as well as degradation of the inhibitor IκBα, with concomitant increase in the accumulation of carbonylated proteins, mimicking responses seen in T cells from the elderly. Pretreatment with an antioxidant, NAC could override pro-oxidant-mediated, but not age-associated decrease in both 20S proteasomal activities. These results suggest that the decrease in proteasomal activities observed during aging may be secondary to oxidative stress and underlie immune senescence.
Keywords: Aging, proteasome, oxidative stress, GSH, ROS, T lymphocytes, immune-dysregulation, immune senescence
Introduction
Aging is characterized by a ubiquitous decline in the functional capacity of various physiological compartments including the immune system. Previous studies have clearly demonstrated that aging is accompanied by deficits in activation-induced T cell responses such as of IL-2R expression, IL-2 secretion and proliferation [1–8]. We have previously demonstrated that decreased induction of the transcription factor NFκB, contributes to lowered induction of IL-2R during aging [4,9]. Additional studies demonstrated this decline in NFκB to be attributable to a central defect in the proteolytic activity of the 26S proteasome [4,7,10]. To further delineate the basis for the decline in proteasomal proteolysis, we have now focused our efforts on the role of oxidative stress and the impact of redox regulation on the 26S proteasome function during aging. The 26S proteasome, a multi-subunit protease complex, consists of a 20S catalytic core flanked by 19S regulatory subunits. The 20S core is a barrel-like particle consisting of a stack of four rings made up of two outer α-rings and two inner β-rings. The two inner β- rings contain three pairs of active sites (β1, β2, and β5) that perform distinct proteolytic activities namely peptidyl glutamyl peptide hydrolyzing (or caspase-like), trypsin-like and chymotrypsin-like activity, for cleavage after acidic, basic, and hydrophobic amino acids, respectively [11–13].
Besides targeted degradation of regulatory proteins, an important function of the proteasome is the degradation of oxidized and aberrant proteins [14–17]. Increased accumulation of highly oxidized and cross-linked protein aggregates within the cell observed during aging has been attributed to decreased proteasome function [18,19]. Indeed, both 20S and 26S proteasomes show age-related decline in their enzymatic activities in most cell types ranging from T lymphocytes to neurons [4,10,20–22]. Studies by Chondrogianni et al revealed that, while the inhibition of proteasome induced a senescent phenotype, stable expression of β1 or β5 catalytic subunits in WI38 immortalized fibroblasts and HL60 leukemic cell line results in increased proteasomal activities and cell survival following treatment with oxidants [23]. Additionally, a recent study by the same group demonstrated that over-expression of β5 subunit in primary IMR90 human fibroblasts delays replicative senescence by 4–5 population doublings [24]. Taken together, these studies underscore the importance of proteasome in the progression of the senescent phenotype by regulating cell survival and protection from oxidative stress.
While several studies have now documented declines in proteolytic function of the proteasome during advancing age, few studies, if any, have focused on the underlying basis for this decline. Oxidative stress has often been implicated in the functional loss accompanying aging, more specifically the involvement of thiols in T cell responsiveness to external stimuli [25–29]. However, there is little evidence for the effect of oxidative stress on proteasomal activities in primary human T lymphocytes. The free radical theory of aging postulates that the ubiquitous and progressive decline in the functional capacity of cells during aging is a consequence of oxidative damage caused by ROS [30]. Thus, the proteasome by virtue of being the organelle responsible for the removal of oxidatively modified proteins may itself be a direct target of oxidative stress during aging. We therefore investigated the effect of oxidative stress on proteasomal catalytic activity in T lymphocytes from young and elderly donors.
We report here that upon exposure to pro-oxidant conditions, T cells from young donors mimic responses seen under basal conditions in T cells from elderly. These include a decrease in GSH content, increased accumulation of carbonylated proteins, increased generation of ROS and a decline in proteasomal catalytic activities. Furthermore, pretreatment with N-acetyl cysteine (NAC), afforded protection against pro-oxidant-induced, but not age-associated decrease in proteasomal activities. The difference in the effect of NAC may reveal alterations induced by acute, as opposed to chronic oxidative stress occurring throughout the life of an individual. Thus, modulation of proteasome function by altered redox status may contribute to immune dysfunction in senescence.
Materials and Methods
Reagents
Antibodies to CD19 and human IgM were obtained from Southern Biotech Associates (Birmingham, AL). Anti-CD3 was obtained from Zymed (San Francisco, CA); anti-CD28 and anti-IκBα were obtained from Santa Cruz Biotech (Santa Cruz, CA). Anti-di-nitro-phenyl (DNP) was obtained from Molecular Probes (Eugene, OR) and anti-IgG coupled to horseradish peroxidase was obtained from BD-Transduction Laboratories (Lexington, KY). Enhanced chemiluminescence (ECL) reagents were from Amersham (Arlington Heights, IL). Luciferase reporter assay kit was purchased from Promega (Madison, WI). 2, 7-dichlorodihydrofluorescein diacetate (DCHF-DA) was obtained from Molecular Probes (Eugene, OR). TNF-α was obtained from Upstate Biotechnology Inc, (Lake Placid, NY). Proteasome IP kit was from EMD Biosciences Inc, (San Diego, Ca). Ada-lys-(Biotnyl)-(Ahx)3-(Leu) 3-Vinyl sulfone, was from Biomol, (Plymouth Meeting, PA).
All fine chemicals, unless otherwise mentioned, were obtained from Sigma Chemical Co. (St Louis, MO). Succinyl-leu-leu-val-tyr-4-amido-methyl-coumarin (Suc-LLVY-AMC) was from Bachem Biosciences (King of Prussia, PA). Electrophoresis supplies were from BioRad (Richmond, CA). ILU-18 cell line was obtained from Dr. Charles O’ Brien (UAMS) [31].
Human subjects
Peripheral blood was obtained by venipuncture from healthy individuals. Young donors were 21–30 years of age and elderly donors were 65–80 years of age. Our donor population consisted of an equal representation of males and females. Both young and elderly donors were in good physical and mental health, had no apparent illness as suggested by an elaborate screening history and were on no medication directly impacting the immune function during the course of this study. A minimum of five donor pairs were used in each experiment. The protocol for blood draw from human subjects was approved by the IRB (UAMS) and blood was drawn at the local General Clinical Research Clinic (GCRC).
T lymphocyte isolation
Peripheral blood mononuclear cells (PBMC) were obtained and T lymphocytes were enriched as previously described [32]. Briefly, PBMCs obtained from young and elderly donors were subjected to panning using anti-human IgM on plastic petri dishes to obtain partially purified T lymphocytes. These cells were then subject to a second round of negative selection using anti-CD19 magnetic beads. Isolated T cells were found to be 90–95% CD3 positive as determined by flow cytometry using FITC-labeled CD3 antibody.
Measurement of T lymphocyte proliferation
T lymphocytes (1×105/100μl/well) were stimulated with immobilized antibody to CD3 and CD28 (1μg/ml) for 48 h at 37°C in a 96-well plate. Radio-labeled 3H-thymidine (1 μCi/well) was added to the cells during the last 6 h of culture, and incorporated radioactivity was measured using a dry-matrix beta counter (Packard, Pangbourne, UK). Proliferation index was calculated as a ratio of counts obtained in anti-(CD3+ CD28) activated cultures to those in untreated control cultures.
Measurement of intracellular GSH
Intracellular GSH levels were assayed as described [33]. Briefly, 100 μl of ice-cold lysis buffer was added to T cells (1×106), incubated on ice for 10 min, and centrifuged at 12,000×g for 10 min in a microfuge. To this preparation, 2 μl of 25 mM monochlorobimane (MCB) and 2 μl of a 50U/ml glutathione-S-transferase (GST) stock, were added and incubated at 37°C for 30 min. At the end of incubation, fluorescence was measured using a fluorescence plate reader (TECAN, NC.) at an excitation wavelength of 340 nm and emission wavelength of 465 nm. Values obtained in the absence of GST and MCB served as negative controls.
Measurement of intracellular ROS using DCHF-DA
T lymphocytes (1×106/ml) were incubated with 10 μM DCHF-DA at 37°C for 30 min in dark [34]. At the end of incubation, cells were washed and resuspended in PBS. ROS generation was monitored following addition of BSO (200 μM) and detected using a spectrofluorometer (Perkin Elmer Corporation, Norwalk, CT) at an excitation wavelength of 480 nm and emission wavelength of 525 nm. Generation of ROS is expressed as mean fluorescence intensity (MFI) at various time points.
Determination of 20S proteasome catalytic activities
T cells were lysed using proteasome lysis buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, and 250 mM sucrose followed by centrifugation at 12,000×g for 10 min [4]. The supernatant was then subjected to ultra centrifugation at 100,000×g for 1 h at 4οC. 20S proteasome-enriched fraction (50 μg protein) was incubated in 200 μl of assay buffer containing 50 mM Tris-HCl (pH 7.8), 10 mM MgCl2, and 1 mM dithiothreitol (DTT) for 1 h at 37°C in the absence of ATP. Fluorogenic peptide substrate (Suc-LLVY-AMC, Bz-R-AMC or N-LLE-βNA, 40 μM) was added, and incubated for 30 min at 37°C. The reaction was quenched with 1 ml of ice-cold ethanol. Hydrolysis of the fluorogenic peptide was determined by measuring fluorescence using spectrofluorometer at an excitation wavelength of 380 nm and emission wavelength of 440 nm for AMC substrates, and at an excitation wavelength of 335 nm and emission wavelength of 410 nm for β-NA substrate. A proteasome specific inhibitor was employed to ensure specificity of the assays.
Determination of 26S proteasome activities
26S proteasome-enriched fractions were prepared as described earlier [4] or by immuno-precipitation employing the proteasome isolation kit (EMD Biosciences, CA). Briefly, T cells were lysed using proteasome lysis buffer containing 50 mM Tris-HCl (pH 7.4), 5 mM MgCl2, 250 mM sucrose and 2 mM ATP and subjected to centrifugation at 12,000×g for 10 min. The supernatant was subsequently subjected to ultra centrifugation at 100,000×g for 6 h at 4οC. At the end of centrifugation, the pellet fraction containing 26S proteasome was resuspended in buffer containing 50 mM Tris–HCl (pH 7.8), 10 mM MgCl2, 20% glycerol and 2 mM ATP and subjected to immuno-precipitation using the isolation kit, per manufacturer’s protocol. Proteasome enriched preparations were Western blotted, and assayed for catalytic activity in 3 pairs of young and elderly donors, using 5μM lactacystin or MG132 to establish specificity (Fig. 2A[suppl.] & 2B[suppl.]). Proteasome specific catalytic activity using fluorogenic substrate was found to be similar in enriched preparations of proteasome obtained either by immuno-precipitation or stepwise ultracentrifugation. 26S enriched fraction or immune complexes (50 μg protein) were incubated in 200 μl of assay buffer containing 50 mM Tris–HCl (pH 7.8), 10 mMMgCl2, 1 mM DTT, and 0.5 mM of the fluorogenic peptide substrate, in the presence or absence of 2 mM ATP (dose determined to be maximal for activity;. Fig. 1 [suppl.]). After incubation for 1 h at 37°C the reaction was quenched with 1 ml of ice-cold ethanol. Hydrolysis of fluorogenic peptide substrates and specificity of the assay were determined as described [4]. ATP specific activity was obtained by using apyrase, and proteasome specific activity was calculated following subtraction of activity obtained in the presence of apyrase and was completely inhibited in the presence of proteasome inhibitor MG132 or LLnL.
Fig. 2. Increased protein carbonylation during oxidative stress and aging.
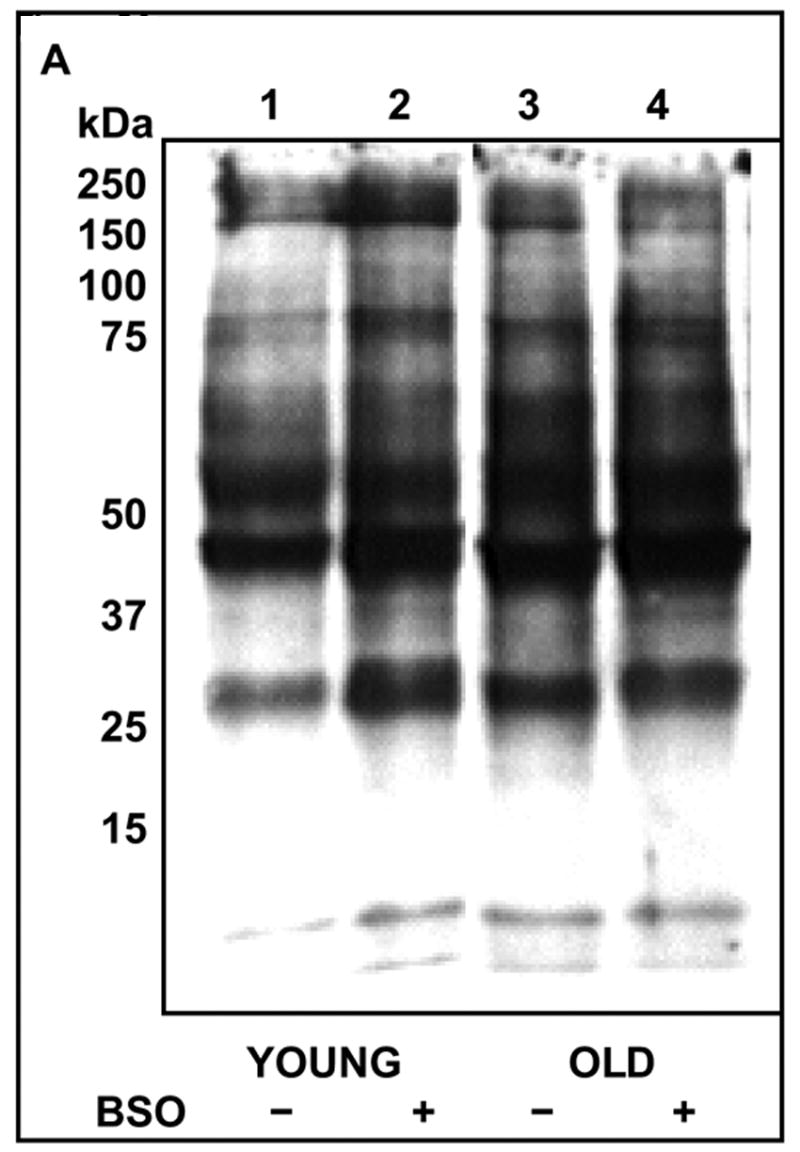

A: T cells from young (Y) and elderly (O) donors were either exposed to 200 μM BSO for 24 h (+) or left untreated (−). Cytosolic lysates obtained from these cells were derivatized using 10 mM DNPH, and subjected to SDS-PAGE, followed by Western blotting using anti-DNP antibody and detected by ECL. Data obtained from a representative donor pair is presented.
B: Mean integrated density ± S.E of total carbonylated proteins obtained by densitometry scanning from 8 independent experiments is presented. * Denotes significance at p < 0.05, between the groups indicated.
Fig 1. Treatment with BSO depletes intracellular GSH and induces generation of ROS in T lymphocytes from young donors.

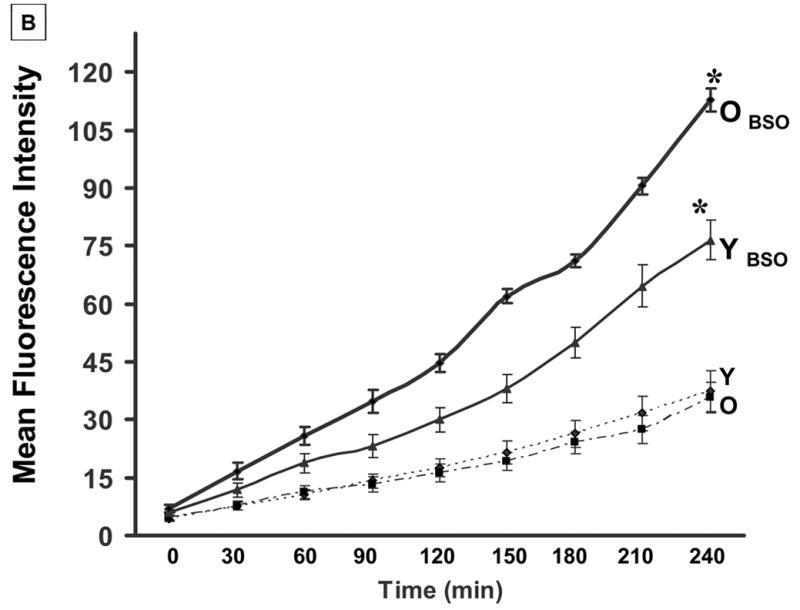
A: T cells from young (Y) and elderly (O) donors were either exposed to 200 μM BSO (+) for 24 h or left untreated (−). GSH levels were measured as described, from triplicate cultures. Results are presented as mean fluorescence intensity (MFI) and are representative of a minimum of 5 independent experiments.
B: T cells from young and elderly donors were loaded with 10 μM DCHF-DA at 37°C for 30 min in the dark. At the end of the incubation, cells were washed and resuspended in PBS. Generation of ROS was detected at different time points, following addition of BSO, using a spectrofluorometer (excitation λ 480 nm and emission λ 525nm) and values are expressed as MFI. * Denotes statistical significance at p < 0.001, between the groups, as indicated.
Proteasome specific activity was also evaluated in Jurkat T cells by employing a novel biotinylated proteasome activity profiling probe [35]. Jurkat T cells (20 × 106 ), either untreated or treated with 200μM BSO for 24h, with or with out 5mM NAC pretreatment, were washed twice with cold phosphate-buffered saline, pelleted by centrifugation, and lysed in buffer containing 50mM Tris-HCl (pH 7.4), 5mM MgCl2, 250mM sucrose and 2mM ATP. Lysates from Jurkat cells pre-treated with 5μM Lactacystin for 2 h and labeled with the biotinylated probe served as a specificity control. Cell lysates equalized for protein (30μg) were labeled with 5μM biotinylated probe in a reaction buffer containing 50mM Tris-HCl (pH 7.4), 5mM MgCl2, 2mM ATP and 2mM DTT for 2h at 37 C. Labeling reactions were quenched by the addition of SDS-sample buffer and boiling for 5 min. Samples were resolved by 12% SDS-PAGE, transferred to nitrocellulose membranes, immunoblotted with streptavidin conjugated to horseradish peroxidase (HRP) followed by ECL.
Detection of activation-induced IκBα degradation by Western blotting
Cytosolic extracts for Western blotting were prepared by homogenization of cells in lysis buffer containing 1 mM Hepes, 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium orthovanadate, 0.5% NP-40, 0.5 mM DTT, 0.5 mM phenyl methyl sulphonyl fluoride, and 10 μg/ml each of aprotinin, leupeptin, and soybean trypsin inhibitor [4]. Protein content of cytosolic extracts was determined using Biorad protein assay. Cell lysates equalized for protein (30 μg), were resolved by 10 % SDS- PAGE, transferred to nitrocellulose membrane, immunoblotted with anti-IκBα antibody and detected using anti-IgG coupled to HRP followed by ECL.
Detection of carbonylated protein by Western Blotting
Carbonylated proteins were detected using OxyBlot™ protein oxidation detection kit (Chemicon, Temecula, CA), per manufacturer’s protocol. Briefly, T cells were lysed in 6 % SDS and 2, 4-dinitrophenyl-hydrazine (DNPH) was added to derivatize the carbonyl groups in the protein side chains to 2, 4-DNP-hydrazone. DNP-derivatized proteins were resolved by 12 % SDS-PAGE and detected by Western blotting using anti-DNP antibody followed by anti-rabbit-IgG-HRP and ECL.
Measurement of NFκB- dependent transcriptional activity using luciferase reporter assay
ILU-18 cells were derived from a bone marrow stromal cell line called +/+ LDA.11 and transfected with murine IL-6 minigene containing the luciferase promoter and 3′UTR. ILU- 18 cells were either left untreated or treated with TNF-α (20 ng/ml) for 24 h at 37°C. Lysates were prepared using reporter lysis buffer and luciferase reporter activity was measured employing 50μg protein, per manufacturer’s protocol (Promega, Madison, WI).
Statistical analysis
A minimum of 5 samples per age group was analyzed for each facet of the study. Statistical analysis was performed using ANOVA (one-way or two- way, wherever appropriate), followed by Student Newman-Kuel test for multiple pair-wise comparisons. Probability of p<0.05 was considered to be significant.
Results
1. Pro-oxidant treatment decreases intracellular levels of GSH and up regulates ROS generation in T lymphocytes
As levels of GSH and induction of ROS are counter-regulated in the cell, we first examined the basal levels of GSH in T cells from young and elderly donors. Our results demonstrate that constitutive levels of GSH in T cells from the elderly were significantly lower than those in young donors, consistent with previous reports in murine T cells [36]. Upon exposure to a mild pro-oxidant, BSO for 24 h, T lymphocytes obtained from young donors exhibited a significant decrease in the levels of GSH at 24 h, when compared to untreated cells (Fig 1a). T cells from elderly donors exposed to BSO failed to further down regulate GSH levels. In contrast, basal levels of ROS were not affected by the age of the donor (Fig 1b). This is because DCF-DA, the probe used in our analysis, fluoresces on exposure to oxidants, especially H2O2 and peroxides. However, immediately upon exposure to BSO, a significant increase in the generation of ROS could be detected in cells from both young and elderly donors, with the increase being significantly higher in T cells from the elderly (Fig 1b).
2. Age-associated increase in protein carbonylation could be mimicked in T cells from young donors, upon exposure to a pro-oxidant
As protein carbonylation is a characteristic feature of oxidative stress and has been shown to occur with advancing age in various tissues, we assessed the effect of age on protein carbonylation in T cells. Increased protein carbonylation could be detected in T cells from elderly donors when compared to those from young donors (Fig 2). Upon exposure to a mild pro-oxidant, the levels of carbonylated proteins increased significantly in T cells from young donors, mimicking levels similar to those seen under basal conditions in cells from the elderly. In contrast, pro-oxidant treatment failed to induce a similar increase in carbonylated proteins, in T cells from the elderly.
3. Exposure to pro-oxidant down-regulates ATP-independent proteasomal activity while treatment with an anti-oxidant ameliorates oxidative stress induced loss in catalytic activity
We next investigated the effect of oxidative stress on 20S proteasome-associated enzymatic activities in T cells from both young and elderly donors. Exposure to oxidative stress resulted in a significant decline in 20S proteasomal peptidyl glutamyl peptide hydrolyzing or caspase-like (Fig 3a) and chymotrypsin-like (Fig 3b) activity in T cells obtained from young donors when compared to those seen under basal conditions. Our results demonstrate that 20S chymotryptic and caspase-like activities are significantly decreased under basal condition in T cells from the elderly. However, exposure to pro-oxidant condition failed to further down-regulate proteasomal activities in cells from the elderly. Thus, the decline in 20S caspase-like and chymotryptic activities observed in pro-oxidant treated T cells from young donors mimicked the decline observed in T cells from elderly (Fig 3a and 3b). We further confirmed the oxidative stress-induced decline in proteasomal proteolysis occurring in T cells, by employing a novel active site probe for proteasome in Jurkat T cells. Jurkat T cells either untreated or exposed to BSO (24h) with or without NAC pretreatment, were lysed and lysates were incubated with activity profiling probe for 2 h. Jurkat cells pretreated with 5μM lactacystin served as specificity control. As seen in fig.3c, exposure to BSO down regulated proteasome specific activity as exemplified by β5 and β1 subunits, and NAC pretreatment overrode BSO-induced inhibition in activity. This is similar to the data seen in T cells obtained from young donors. Pretreatment of T cells with an anti-oxidant, NAC, prior to pro-oxidant exposure, helped override oxidative stress-induced decline in both the post-acidic and chymotryptic activities in cells from young donors and Jurkat T cells. Additionally, similar to the observation obtained following exposure to the pro-oxidant, neither NAC nor a combination of NAC and BSO treatment induced any significant change in the 20S proteasomal activities in T cells obtained from elderly donors (Fig 3).
Fig. 3. Treatment with anti-oxidant ameliorates pro-oxidant-mediated decline in 20S proteasomal activities, but fails to restore age-associated loss in activity.
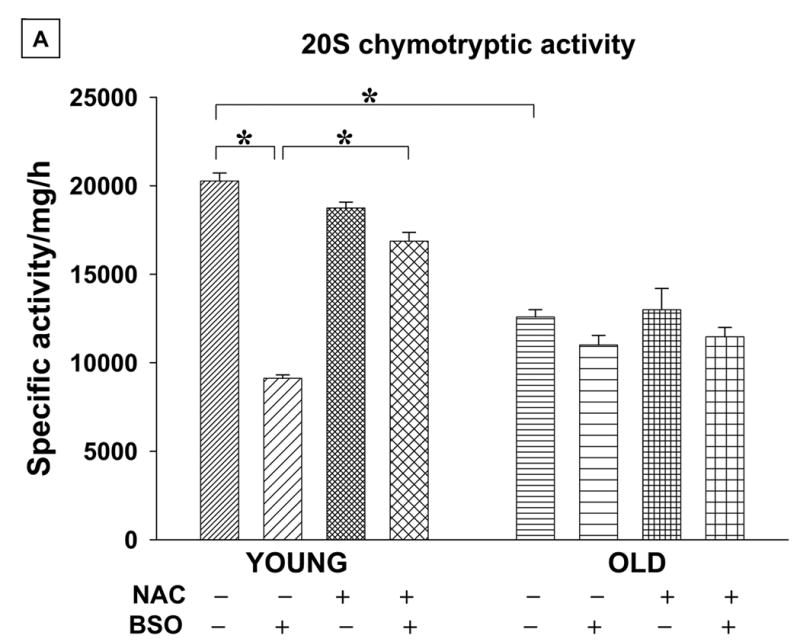


A & B. T cells (50x 106) from young (Y) and elderly (O) donors were either pretreated with 30 mM NAC for 2 h or left untreated (−) for 24 h. At the end of pretreatment cells were exposed to 200μM BSO (+). Enriched 20S proteasome fractions from these cells, (50μg) were used in proteolytic activity assay evaluated using specific fluorogenic substrates, Suc-LLVY-AMC and N-LLE-βNA for chymotryptic and post-acidic activity, respectively. Data are presented as specific activity/mg/hr ± SE obtained from a minimum of 5 donor pairs. * Denotes statistical significance at p < 0.001, between the groups indicated. Fig.3.A: Represents results of chymotryptic activity and Fig.3.B: represents results of peptidyl glutamyl hydrolytic activity.
C. BSO down regulates 20S proteasome activity in Jurkat T cells assayed employing a novel biotinylated proteasome active site probe. Lysates obtained from untreated Jurkat cells, or those exposed to either 200 μM BSO for 24 h or pretreated with NAC (5mM) for 2h followed by treatment with BSO for 24h, were labeled with 5μM biotinylated proteasome – profiling probe for 2h at 37 C. Labeled lysates were resolved using SDS-PAGE on 12% gels and immunoblotted using streptavidin conjugated to horse-radish peroxidase and ECL. Lysates obtained from cells treated with 5μM Lactacystin (Lacta.) for 2 h, prior to labeling with the active site probe, is included as specificity control.
4. Exposure to pro-oxidant down regulates catalytic activities associated with the 26S proteasome
Previous studies from our laboratory have demonstrated significant decrease in proteasomal catalytic activity in T cells from elderly donors. However, the role of oxidative stress in the regulation of 26S proteasome in human T lymphocytes remains largely unknown. We therefore investigated the effect of pro-oxidant exposure on ATP-stimulatable enzymatic activities associated with 26S proteasome in T cells from both young and elderly human donors. Interestingly, while T cells from young donors demonstrated robust proteasomal proteolytic activity under basal conditions, exposure to the pro-oxidant, demonstrated significant loss in chymotryptic (Fig 4a) and caspase-like hydrolyzing activities (Fig 4b) similar to that observed under basal conditions in cells from elderly donors. However, it is interesting to note that the trypsin-like activity in T cells from young donors was resistant to oxidative stress-mediated decline in activity (Fig 4c). In contrast, basal levels of all the three major catalytic activities were significantly down-regulated in T cells from the elderly, the extent of decline in function was maximum for the chymotryptic activity (69%), with caspase-like hydrolyzing and tryptic activity demonstrating between 40–50% loss in activity when compared to that observed in untreated T cells from young donors (Fig 4a, 4b and 4c respectively). However, exposure to pro-oxidant failed to further down regulate any of the ATP-stimulatable proteasomal activities in T cells from elderly donors (Fig 4).
Fig. 4. 26S proteasome associated catalytic activities are down regulated in T cells from the elderly and following exposure to pro-oxidant treatment.



T cells (50×106) from young (Y) and elderly (O) donors were exposed to 200 μM BSO (+) or left untreated (−) for 24 h. At the end of treatment, 50 μg of enriched 26S proteasome fraction were assayed for chymotryptic activity (A); peptidyl glutamyl hydrolytic activity (B); and tryptic activity (C), using, Suc-LLVY-AMC, N-LLE-βNA, Bz-R-AMC; respectively. Data are presented as specific activity/mg/hr ± SE obtained from a minimum of 5 donor pairs. * Denotes statistical significance at p < 0.001, between the groups indicated.
5. Oxidative stress down regulates TNF-α–induced degradation of IκBα
As previous studies from our laboratory have clearly demonstrated a vital role for the functional loss of 26S proteasomal activity in age-associated decline in NFκB induction in T cells, we examined the effect of pro-oxidant treatment on activation-induced degradation of IκBα (inhibitor of NFκ B), as a direct correlate of 26S proteasome function. Treatment with TNF-α resulted in lower levels of IκBα when compared to untreated controls, indicating activation-induced degradation in T cells from young, but not in cells obtained from the elderly donors (Fig 5a, b), consistent with our previously published results [7]. However, upon pro-oxidant exposure, TNF-α treatment failed to induce degradation of IκBα, mimicking lowered degradation observed in T cells from the elderly (Fig 5a, b). Interestingly, pro-oxidant exposure failed to impact on IκBα levels following TNF-α activation in T cells from the elderly (Fig 5).
Fig. 5. TNF-α mediated degradation of IκB-α is affected by aging and pro-oxidant treatment.


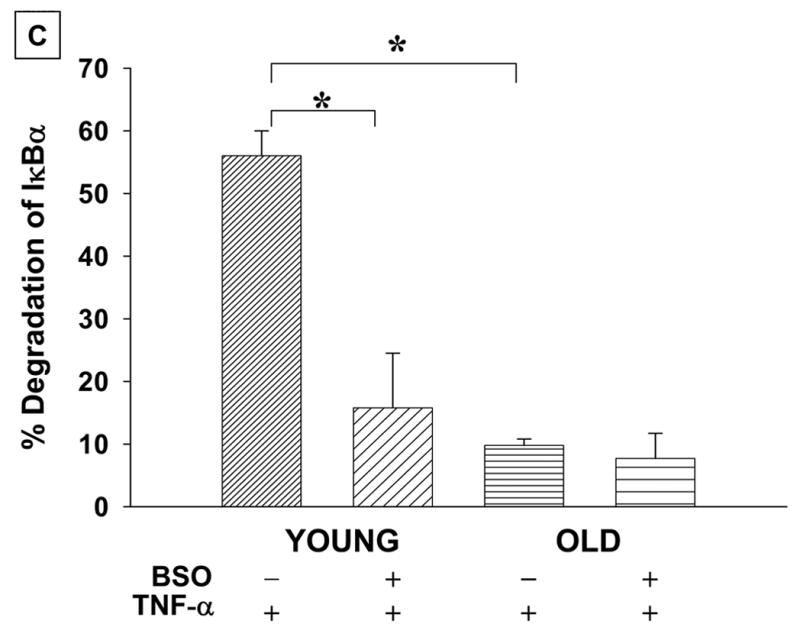
A: T cells from young (Y) and elderly (O) donors were either exposed to 200 μM BSO (+) for 24 h or left untreated (−). At the end of 24 h, cells were either treated with 10 ng/ml TNF-α for 10 min (+) or left without treatment (−). Cytosolic lysates were equalized for protein (30μg) and subjected to SDS-PAGE, followed by Western Blotting using antibody to IκBα and detected by ECL. Data obtained from a representative donor pair is presented. The blot was stripped and probed with antibody to β-actin to demonstrate equal protein loading in all the lanes.
B: Quantitation of IκBα degradation as detected by the disappearance of the specific band in Western blots was carried out by densitometry scanning, from 7 independent donor pairs.
C: Degradation of IκBα is presented as percent decrease in levels obtained in TNF-α treated cells when compared to untreated controls. Values represent Mean ± SE of percent degradation obtained from independently conducted experiments 7 donor pairs.
* Denotes statistical significance at p <0.001, between the indicated groups.
6. Pro-oxidant treatment down regulates NFκB-dependent transcriptional activity
To further analyze whether the observed decrease in IκBα degradation following exposure to the pro-oxidant impacts on transcriptional activity of NFκB, we next assayed NFκB-dependent transcriptional activity using a NFκB-driven reporter assay. As shown in Fig. 6, pro-oxidant treatment significantly decreased TNF-α -induced NFκB-dependent transcriptional activity by 52%, when compared to the activity in cells treated with TNF-α alone. Pretreatment with NAC inhibited the pro-oxidant-mediated loss in transcriptional activity. It should be noted that treatment with pro-oxidant alone in the absence of any stimulus consistently induced NFκB-dependent transcriptional activity at relatively low levels (Fig 6). Treatment with anti-oxidant prior to TNF-α treatment suppressed NFκB-dependent transcriptional activity, suggesting that TNF-α mediated induction of NFκB transcriptional activity involves the participation of oxidant sensitive “steps” along the pathway. In fact, recent studies have demonstrated a central role for oxidant in TNF-α mediated induction of NFκB [37].
Fig. 6. Pretreatment with pro-oxidant down-regulates NFκB-dependent reporter activity induced by TNF-α.
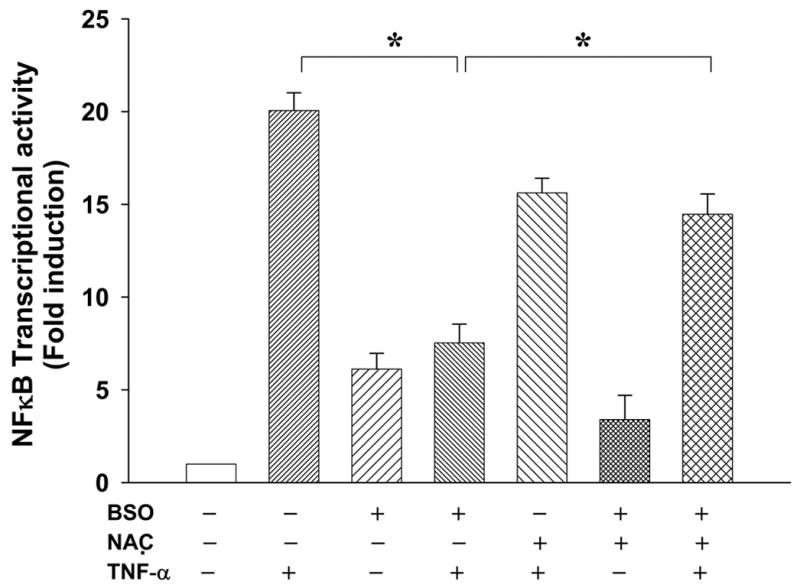
ILU-18 cells were either exposed to 200 μM BSO (+) for 24 h or left untreated (−). At the end of 24 h, the cells were activated with 10 ng/ml of TNF-α for 24 h (+), or left untreated (−). To override the effect of pro-oxidant exposure; cells were also pretreated for 2 h with NAC, followed by BSO and TNF-α as indicated. Luciferase reporter assay was performed to determine NFκB-dependent transcriptional activity, as per manufacturer‘s protocol. * Denotes statistical significance at p < 0.001 between indicated groups.
7. Pro-oxidant treatment down regulates activation-induced T cell proliferation
We next assessed the effect of pro-oxidant treatment on T cell functional response by assaying the proliferative potential of T cells to mitogenic stimulation. As shown in Fig 7, exposure to pro-oxidant, prior to activation, decreased the proliferative ability of T cells from young donors by 40%, when compared to control cultures similarly activated in the absence of pro-oxidant, mimicking responses seen upon mitogenic treatment of T cells from the elderly. Exposure to pro-oxidant had no further dampening effect on the proliferative ability of T cells obtained from the elderly. Thus, T cells from young donors, when exposed to pro-oxidant stimuli, demonstrated a loss in proliferative potential mimicking responses observed in activated T cells from the elderly.
Fig.7. Aging and oxidative stress down-regulates activation-induced T cell proliferation.

T cells from young (Y) and elderly (O) donors were either exposed to 200 μM BSO (+) for 24 h or left untreated (−). At the end of incubation, cells (1x 106) were stimulated with plate- bound 1μg/ml anti-(CD3+ CD28) for 48 h at 37ºC. Proliferation was measured by 3H-thymidine uptake for the last 6 h of culture. Data are presented as proliferative index ± SE from 8 independent experiments. * Denotes statistical significance at p < 0.001, between groups.
Discussion
As aging appears to be inherently associated with oxidative stress, an important role for ROS is becoming increasingly appreciated in T cell hypo-responsiveness during senescence. Oxidative stress-induced modifications in proteins have been documented in different cell types [21,22,38]. Consistent with this observation, our results demonstrate that increased protein carbonylation seen in T lymphocytes from elderly donors could be mimicked in T cells from young donors following exposure to pro-oxidant. As the proteasome is largely implicated in the removal of oxidized proteins [18–22,39], accumulation of carbonylated proteins during aging and following pro-oxidant exposure suggests that proteasome may itself be a target of oxidative stress. Indeed, cross-linked proteins formed by prolonged oxidation and/or HNE modifications have previously been shown to inhibit the proteasome [16]. In addition, recent studies have demonstrated that proteasome is susceptible to free radical-induced modification and inactivation during coronary occlusion/reperfusion [21]. These results highlight an interesting paradox; the protease responsible for the removal of oxidized proteins is itself a target of oxidative modification. Thus, this study was undertaken to investigate the effect of oxidative stress-induced alteration in proteasomal activity as one of the possible mechanisms that contribute to age-associated T cell dysfunction.
We demonstrate here that exposure of T cells from young donors to pro-oxidant conditions, such as acute treatment with BSO, results in the loss of proteasomal activities that impact T cell function, mimicking hypo- responsiveness observed in T cells from the elderly. Thus, it appears that oxidative stress-mediated defects in proteasome function may well contribute to age-associated defects in T cell responses. Further, pretreatment with an anti-oxidant, NAC, afforded protection against oxidative stress-induced loss in the catalytic activity associated with 20S proteasome in the young, but not in the elderly. NAC pretreatment failed to afford complete protection of the 26S proteasome, as seen in the reporter assays, suggesting that the regulatory subunits of the 19S may be highly sensitive targets of such oxidative insults during aging. Additionally, failure of NAC to completely override proteasomal defects in T cells from the elderly as opposed to T cells from young donors exposed to pro-oxidant may indicate that acute exposure, but not chronic exposure to oxidative stress, is reversible. Alternatively, it may indicate that short term treatment with NAC, is not sufficient to override the loss induced by chronic exposure to oxidants. Additionally, it should be noted that 5mM NAC employed in our assays is capable of partial restoration of pro-oxidant-mediated effects, but higher concentration of NAC effectively inhibits 26S proteasome. Previous studies have implicated a role for NAC in modulating 19S rather than 20S catalytic subunits [40]. However, further studies are needed to directly demonstrate 19S as targets of NAC treatment. It has also been suggested that NAC may interfere with 26S activity by modifying ubiquitination of targets directed to the proteasome by modulating cellular thiol levels. However, this may not be the case in our study since we employed fluorogenic substrates that do not require ubiquitination for degradation, but nevertheless, the lack of effect on 20S activity as opposed to 26S mediated hydrolysis implicates a role for ATPase domains of 19S as targets of inhibition by NAC.
Diminished clearance of oxidized proteins during aging and age-associated diseases underscores the importance of proteasomes in the maintenance of cellular homeostasis. Results presented in this paper confirm that both ATP-stimulatable as well as ATP-independent proteasomal catalytic activities are compromised during human aging, with chymotrypsin-like activity being most affected in T lymphocytes. These results are in agreement with studies reported employing muscle lysates from LOU rat [20], but, in contrast, to the results obtained in the liver by Shibatani and coworkers, who found that basal peptidase activities were not affected, while SDS-activated 20S chymotrypsin-like and trypsin-like activities were increased and caspase-like activity was decreased with advancing age [41]. This discrepancy in data may be attributable to the specificity of the tissues employed in the studies and to the relative stoichiometry of constitutive and immuno-proteasome subunits within the catalytic core of the proteasome in these tissues.
Oxidatively modified proteins have been demonstrated to be preferentially degraded in vitro by the 20S proteasome in an ATP-independent fashion [14,15,20,39]. While earlier studies employing fibroblasts have suggested that the 20S proteasome might be resistant to age-associated or oxidative stress-induced modifications [18,42] recent studies employing cardiac muscles and liver from aged rodent cohorts, demonstrate significant decline in 20S proteasomal activities [21,43]. Our current results with 20S proteasomes preparations which demonstrate chymotrypsin-like activity to be most sensitive to age-related modifications is in line with our previously reported observation obtained with 26S proteasomes, as well as, data reported by Carrard et al, employing peripheral blood lymphocytes from human subjects [4,22]. Studies by Carrard et al have also demonstrated that β-subunits of 20S proteasome are prone to glycation, HNE modification and ubiquitination during aging [22], with the β7 and the β5i subunits being the most sensitive targets for such modifications. Incidentally, the chymotryptic activity is mediated by the β5 subunit in the proteasome, which is replaced by the β5i subunit in the immuno-proteasome [11]. Hence, it would not be far fetched to speculate that the observed loss in the chymotrypsin-like activity of the proteasome during oxidative stress and aging may well be attributed to such modifications in T lymphocytes. Furthermore, it is likely, that depending on the constitution of its catalytic core, i.e. the ratio of immuno-proteasome to constitutive subunits, susceptibility to exogenous modifiers may well vary. Recent studies by Chondrogianni et al have provided proof of this concept in primary IMR90 human fibroblasts over-expressing β5i catalytic subunits [24].
Pro-oxidant exposure of T cells from young donors via depletion of intracellular GSH resulted in diminished ATP-dependent and independent proteasomal catalytic activities, demonstrating their susceptibility to the altered cellular redox status. While the pro-oxidant treatment resulted in a significant decline in ATP-independent proteasomal activity, it was relatively less sensitive than ATP-dependent catalytic activities, and is consistent with previous studies employing fibroblasts [42].
As activation-induced degradation of IκBα not only serves as an ideal target for regulated turn-over by the proteasome, but is also a critical step in the induction of the transcription factor NFκB, quintessential immune response gene inducer [44]. We therefore analyzed activation-induced IκBα degradation in T cells exposed to oxidative stress. Oxidative stress-induced decrease in proteasomal activity clearly inhibited the degradation of IκBα, thus mimicking age-associated loss in IκBα degradation reported previously [4,7]. This decrease in IκBα degradation, translated into a lower transcriptional activity mediated by NFκB, indicating that oxidative stress-mediated impairment of proteasomal activity impacts on regulatory functions dependent on the proteasome. Although activity mediated by NFκB has been demonstrated to be susceptible to alterations in the intracellular redox status in other cell types, the role of redox remains controversial in T cells [45]. Induction of NFκB activity has been reported in T cells following short-term administration of exogenous ROS [46] by a proteasome independent pathway. Consistent with our observations in pro-oxidant treated ILU-18 cells, chronic oxidative conditions have been shown to decrease NFκB induction in human T cells [47]. As the proteasome is a key regulator of NFκB activity [48], lowered levels of this transcription factor under oxidative conditions may well be due to a decrease in proteasomal activity under such circumstances.
Decline in T cell proliferation following polyclonal activation during aging and oxidative stress may be linked to decreased proteasomal catalytic activities under these conditions. This observation is consistent with the fact that proteasomes play a key role in cell proliferation by regulating the progression of cells from G0 to S phase [49]. Additionally, several targets within the signaling cascade including PLCγ1, intracellular Ca2+, IKK [50,51], as well as lower GSH [52], may contribute to lowered proliferation seen upon pro-oxidant exposure.
Thus, results presented here clearly underscore the central role of oxidative stress in decreased proteasomal proteolytic activity during aging, which may be a contributing factor for T cell hypo-responsiveness. Our results not only corroborate recent studies suggesting that altered redox status of T lymphocytes govern their responses to different stimuli [28,29], but also provide a mechanistic explanation for age-associated loss in T cell functional responses, due to lowered NFκB induction. The finding that oxidative stress may be a forerunner for loss in proteasomal function during aging has far reaching implications, not only for immune response gene induction but also for other regulatory processes, such as cell cycle regulation, as well as, the generation of antigenic peptides, which are important for immune regulation. Thus, interventions that override chronic oxidative stress-mediated alterations in proteasome structure and function or approaches that induce proteasomal subunits may provide valuable information for designing immunomodulatory agents for reversing senescence-induced changes in the immune system.
Supplementary Material
Acknowledgments
This work was supported by Grants provided by NIH RO1AG13081, MO1RR1288, and in part by the University of Arkansas for Medical Sciences Graduate Student Research Fund. We gratefully acknowledge the assistance provided by the General Clinical Research Center, in sample collection, and CAVHS for facilities provided.
Abbreviations
- AMC
amido-methyl-coumarin
- β-NA
beta-naphthylamide
- BSO
Buthionine Sulfoximine
- GSH
glutathione
- IL-2R
Interleukin-2 Receptor
- NAC
N-acetyl-L-cysteine
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fulop T, Jr, Douziech N, Larbi A, Dupuis G. The role of lipid rafts in T lymphocyte signal transduction with aging. Ann N Y Acad Sci. 2002;973:302–304. doi: 10.1111/j.1749-6632.2002.tb04655.x. [DOI] [PubMed] [Google Scholar]
- 2.Ginaldi L, De MM, Modesti M, Loreto F, Corsi MP, Quaglino D. Immunophenotypical changes of T lymphocytes in the elderly. Gerontology. 2000;46:242–248. doi: 10.1159/000022167. [DOI] [PubMed] [Google Scholar]
- 3.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 4.Ponnappan U, Zhong M, Trebilcock GU. Decreased proteasome-mediated degradation in T cells from the elderly: A role in immune senescence. Cell Immunol. 1999;192:167–174. doi: 10.1006/cimm.1998.1418. [DOI] [PubMed] [Google Scholar]
- 5.Sadighi Akha AA, Miller RA. Signal transduction in the aging immune system. Curr Opin Immunol. 2005;17:486–491. doi: 10.1016/j.coi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 7.Trebilcock GU, Ponnappan U. Nuclear factor-kappaB induction in CD45RO+ and CD45RA+ T cell subsets during aging. Mech Ageing Dev. 1998;102:149–163. doi: 10.1016/s0047-6374(97)00160-7. [DOI] [PubMed] [Google Scholar]
- 8.Whisler RL, Karanfilov CI, Newhouse YG, Fox CC, Lakshmanan RR, Liu B. Phosphorylation and coupling of zeta-chains to activated T-cell receptor (TCR)/CD3 complexes from peripheral blood T-cells of elderly humans. Mech Ageing Dev. 1998;105:115–135. doi: 10.1016/s0047-6374(98)00084-0. [DOI] [PubMed] [Google Scholar]
- 9.Trebilcock GU, Ponnappan U. Evidence for lowered induction of nuclear factor kappa B in activated human T lymphocytes during aging. Gerontology. 1996;42:137–146. doi: 10.1159/000213785. [DOI] [PubMed] [Google Scholar]
- 10.Ponnappan U. Ubiquitin-proteasome pathway is compromised in CD45RO+ and CD45RA+ T lymphocyte subsets during aging. Exp Gerontol. 2002;37:359–367. doi: 10.1016/s0531-5565(01)00203-0. [DOI] [PubMed] [Google Scholar]
- 11.Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 12.Djaballah H, Rowe AJ, Harding SE, Rivett AJ. The multicatalytic proteinase complex (proteasome): structure and conformational changes associated with changes in proteolytic activity. Biochem J. 1993;292(Pt 3):857–862. doi: 10.1042/bj2920857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivett AJ. Proteasomes: multicatalytic proteinase complexes. Biochem J. 1993;291(Pt 1):1–10. doi: 10.1042/bj2910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 15.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- 16.Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 17.Rivett AJ. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J Biol Chem. 1985;260:300–305. [PubMed] [Google Scholar]
- 18.Sitte N, Merker K, Von ZT, Grune T. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic Biol Med. 2000;28:701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 19.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 20.Bardag-Gorce F, Farout L, Veyrat-Durebex C, Briand Y, Briand M. Changes in 20S proteasome activity during ageing of the LOU rat. Mol Biol Rep. 1999;26:89–93. doi: 10.1023/a:1006968208077. [DOI] [PubMed] [Google Scholar]
- 21.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 22.Carrard G, Dieu M, Raes M, Toussaint O, Friguet B. Impact of ageing on proteasome structure and function in human lymphocytes. Int J Biochem Cell Biol. 2003;35:728–739. doi: 10.1016/s1357-2725(02)00356-4. [DOI] [PubMed] [Google Scholar]
- 23.Chondrogianni N, Stratford FL, Trougakos IP, Friguet B, Rivett AJ, Gonos ES. Central role of the proteasome in senescence and survival of human fibroblasts: induction of a senescence-like phenotype upon its inhibition and resistance to stress upon its activation. J Biol Chem. 2003;278:28026–28037. doi: 10.1074/jbc.M301048200. [DOI] [PubMed] [Google Scholar]
- 24.Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES. Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem. 2005;280:11840–11850. doi: 10.1074/jbc.M413007200. [DOI] [PubMed] [Google Scholar]
- 25.Cemerski S, van Meerwijk JP, Romagnoli P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur J Immunol. 2003;33:2178–2185. doi: 10.1002/eji.200323898. [DOI] [PubMed] [Google Scholar]
- 26.Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 27.Ginn-Pease ME, Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun. 1996;226:695–702. doi: 10.1006/bbrc.1996.1416. [DOI] [PubMed] [Google Scholar]
- 28.Kanner SB, Kavanagh TJ, Grossmann A, Hu SL, Bolen JB, Rabinovitch PS, Ledbetter JA. Sulfhydryl oxidation down-regulates T-cell signaling and inhibits tyrosine phosphorylation of phospholipase C gamma 1. Proc Natl Acad Sci U S A. 1992;89:300–304. doi: 10.1073/pnas.89.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanagh TJ, Grossmann A, Jaecks EP, Jinneman JC, Eaton DL, Martin GM, Rabinovitch PS. Proliferative capacity of human peripheral blood lymphocytes sorted on the basis of glutathione content. J Cell Physiol. 1990;145:472–480. doi: 10.1002/jcp.1041450312. [DOI] [PubMed] [Google Scholar]
- 30.Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SC, Yamate T, Taguchi Y, Borba VZ, Girasole G, O’Brien CA, Bellido T, Abe E, Manolagas SC. Regulation of the gp80 and gp130 subunits of the IL-6 receptor by sex steroids in the murine bone marrow. J Clin Invest. 1997;100:1980–1990. doi: 10.1172/JCI119729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponnappan U, Trebilcock GU, Zheng MZ. Studies into the effect of tyrosine phosphatase inhibitor phenylarsine oxide on NFkappaB activation in T lymphocytes during aging: evidence for altered IkappaB-alpha phosphorylation and degradation. Exp Gerontol. 1999;34:95–107. doi: 10.1016/s0531-5565(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 33.Kamencic H, Lyon A, Paterson PG, Juurlink BH. Monochlorobimane fluorometric method to measure tissue glutathione. Anal Biochem. 2000;286:35–37. doi: 10.1006/abio.2000.4765. [DOI] [PubMed] [Google Scholar]
- 34.Lahdenpohja N, Savinainen K, Hurme M. Pre-exposure to oxidative stress decreases the nuclear factor-kappa B-dependent transcription in T lymphocytes. J Immunol. 1998;160:1354–1358. [PubMed] [Google Scholar]
- 35.Berkers CR, Verdoes M, Lichtman E, Fiebiger E, Kessler BM, Anderson KC, Ploegh HL, Ovaa H, Galardy PJ. Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nat Methods. 2005;2:357–362. doi: 10.1038/nmeth759. [DOI] [PubMed] [Google Scholar]
- 36.Lohmiller JJ, Roellich KM, Toledano A, Rabinovitch PS, Wolf NS, Grossmann A. Aged murine T-lymphocytes are more resistant to oxidative damage due to the predominance of the cells possessing the memory phenotype. J Gerontol A Biol Sci Med Sci. 1996;51:B132–B140. doi: 10.1093/gerona/51a.2.b132. [DOI] [PubMed] [Google Scholar]
- 37.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulteau AL, Petropoulos I, Friguet B. Age-related alterations of proteasome structure and function in aging epidermis. Exp Gerontol. 2000;35:767–777. doi: 10.1016/s0531-5565(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 39.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in K562 human hematopoietic cells by proteasome. J Biol Chem. 1996;271:15504–15509. doi: 10.1074/jbc.271.26.15504. [DOI] [PubMed] [Google Scholar]
- 40.Pajonk F, Riess K, Sommer A, McBride WH. N-acetyl-L-cysteine inhibits 26S proteasome function: implications for effects on NF-kappaB activation. Free Radic Biol Med. 2002;32:536–543. doi: 10.1016/s0891-5849(02)00743-8. [DOI] [PubMed] [Google Scholar]
- 41.Shibatani T, Nazir M, Ward WF. Alteration of rat liver 20S proteasome activities by age and food restriction. J Gerontol A Biol Sci Med Sci. 1996;51:B316–B322. doi: 10.1093/gerona/51a.5.b316. [DOI] [PubMed] [Google Scholar]
- 42.Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335(Pt 3):637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conconi M, Szweda LI, Levine RL, Stadtman ER, Friguet B. Age-related decline of rat liver multicatalytic proteinase activity and protection from oxidative inactivation by heat-shock protein 90. Arch Biochem Biophys. 1996;331:232–240. doi: 10.1006/abbi.1996.0303. [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 45.Ginn-Pease ME, Whisler RL. Redox signals and NF-kappaB activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 46.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janssen-Heininger YM, Poynter ME, Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 49.Naujokat C, Hoffmann S. Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest. 2002;82:965–980. doi: 10.1097/01.lab.0000022226.23741.37. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S, Bi R, Kim C, Chiplunkar S, Yel L, Gollapudi S. Role of NF-kappaB signaling pathway in increased tumor necrosis factor-alpha-induced apoptosis of lymphocytes in aged humans. Cell Death Differ. 2005;12:177–183. doi: 10.1038/sj.cdd.4401557. [DOI] [PubMed] [Google Scholar]
- 51.Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free Radic Biol Med. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 52.Hadzic T, Li L, Cheng N, Walsh SA, Spitz DR, Knudson CM. The role of low molecular weight thiols in T lymphocyte proliferation and IL-2 secretion. J Immunol. 2005;175:7965–7972. doi: 10.4049/jimmunol.175.12.7965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


