Abstract
Background
TGF-beta is one of the key cytokines implicated in various disease processes including cancer. TGF-beta inhibits growth and promotes apoptosis in normal epithelial cells and in contrast, acts as a pro-tumour cytokine by promoting tumour angiogenesis, immune-escape and metastasis. It is not clear if various actions of TGF-beta on normal and tumour cells are due to differential gene regulations. Hence we studied the regulation of gene expression by TGF-beta in normal and cancer cells.
Results
Using human 19 K cDNA microarrays, we show that 1757 genes are exclusively regulated by TGF-beta in A549 cells in contrast to 733 genes exclusively regulated in HPL1D cells. In addition, 267 genes are commonly regulated in both the cell-lines. Semi-quantitative and real-time qRT-PCR analysis of some genes agrees with the microarray data. In order to identify the signalling pathways that influence TGF-beta mediated gene regulation, we used specific inhibitors of p38 MAP kinase, ERK kinase, JNK kinase and integrin signalling pathways. The data suggest that regulation of majority of the selected genes is dependent on at least one of these pathways and this dependence is cell-type specific. Interestingly, an integrin pathway inhibitor, RGD peptide, significantly affected TGF-beta regulation of Thrombospondin 1 in A549 cells.
Conclusion
These data suggest major differences with respect to TGF-beta mediated gene regulation in normal and transformed cells and significant role of non-canonical TGF-beta pathways in the regulation of many genes by TGF-beta.
Background
TGF-β is a multifunctional cytokine that plays important patho-physiological roles in mammals. There are three mammalian isoforms that are involved in several developmental processes as has been shown by the knock-out mice models [1]. TGF-β has a major role to play in the initiation and progression of cancer. This is supported by several studies which have shown defects in various components of the TGF-β signalling pathway in many cancers [2]. TGF-β has a dual role in carcinogenesis [3]. Initially it acts as a tumour suppressor and causes growth arrest of epithelial cells and cells in the early stages of cancer [4]. But in an established tumour, TGF-β exerts an effect which is favourable for the survival, progression and metastasis of the tumour [5,6] by promoting epithelial-mesenchymal transition (EMT), angiogenesis and escape from immune surveillance [7]. Studies using mouse models have shown that an intact TGF-β signalling is essential for the metastasis of breast cancer [8,9]. These observations indicate that the normal epithelial cells show differential response to TGF-β as compared to the tumour they give rise to. Supporting this, it has been shown that prostate tumour cells show invasion in response to TGF-β and not non-tumourigenic cells [10]. Differential gene expression mediated by TGF-β has been reported in tumour cells and normal cells. For example, in response to TGF-β, tumour cells show increase in the production of proteases and down regulation of the inhibitors of proteases, whereas this is not observed in the normal cells [11-14]. However, there is no clear understanding of the mechanism (s) responsible for differential responses of various cell types to TGF-β. Since a role for TGF-β has been established in several pathological conditions, this pathway is a very attractive target for therapeutic intervention. This requires identification of targets of TGF-β in different cell-types and their mechanism of regulation, particularly in un-transformed and transformed cells. In this study, we show differential regulation of several genes by TGF-β in two different cell-lines, HPL1D and A549 and also propose a significant role for the MAP kinase pathway in TGF-β mediated gene regulations.
Results
Gene expression profiling of HPL1D and A549 cells in response to TGF-β
To identify the TGF-β regulated genes in normal and tumour cells, we chose HPL1D and A549 cells. HPL1D is an immortalized lung epithelial cell-line that is growth inhibited by TGF-β, similar to many epithelial cells [15]. A549 is a lung adenocarcinoma cell-line that has been known to respond to TGF-β treatment [16]. The cells were treated with human recombinant TGF-β 1 for 1, 4 and 12 hours and the RNAs extracted from these cells were used for microarray experiments using human 19 k arrays. Genes which were either up (> 1.3 fold) or down regulated (< 0.33 fold) at any one of the time points have been considered as regulated by TGF-β in the respective cell-line. In HPL1D, 1000 genes were regulated by TGF-β treatment and of these, 917 genes were up regulated and 83 genes were down regulated. In A549, 2024 genes were regulated by TGF-β and of these, 1714 genes were up regulated and 310 genes were down regulated by TGF-β treatment. The log2 transformed data of the genes regulated by TGF-β in HPL1D and A549 cells are shown as hierarchical cluster diagrams (Fig. 1a &1b). The expression profiling data of HPL1D and A549 in response to TGF-β has been submitted to Gene Expression Omnibus (accession number- GSE7436).
Figure 1.
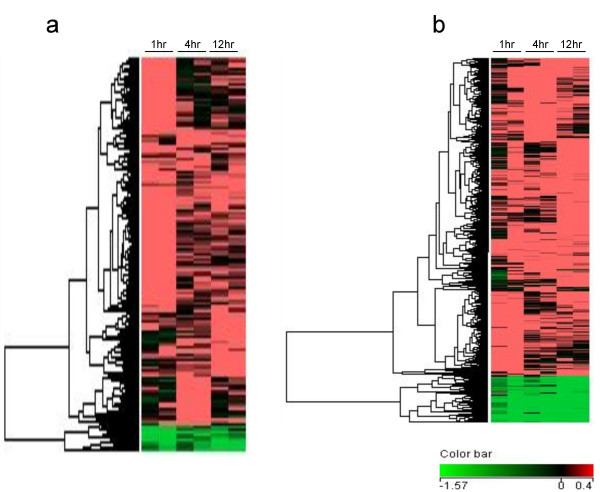
Hierarchical clustering of genes regulated by TGF-β in (a) HPL1D and (b) A549 cells. The genes regulated by TGF-β (described in materials and methods) were clustered using Avadis 3.3 Prophetic software. The data was clustered on rows by one way complete linkage – hierarchical clustering using Euclidean distance metric. Microarrays have been done in duplicate on RNAs isolated from cells after 1, 4 and 12 hours of treatment with TGF-β with paired controls at every time point. The dendrogram on the left shows the different clusters into which the TGF-β regulated genes segregated. Red indicates genes induced by TGF-β treatment, green indicates genes repressed by TGF-β treatment and black suggests no regulation.
Comparison of gene expression profiles of HPL1D and A549 in response to TGF-β
When the gene expression profiles of HPL1D and A549 cell-lines in response to TGF-β 1 were compared, it was found that 267 genes were regulated in both the cell-lines in a similar manner but with different kinetics. The list of genes regulated commonly in both HPL1D and A549 is shown in additional file 1 (Table S1). A hierarchical cluster diagram is shown in figure 2a and a Venn diagram depicting the number of genes commonly and differentially regulated in A549 and HPL1D are shown in figure 2b. 1757 genes are exclusively regulated by TGF-β in A549 cells, (see additional file 2 Table S2) and 733 genes are exclusively regulated by TGF-β in HPL1D cells (see additional file 3, Table S3). Representative clusters of each of the sub-classes of genes are shown in figure 2c.
Figure 2.
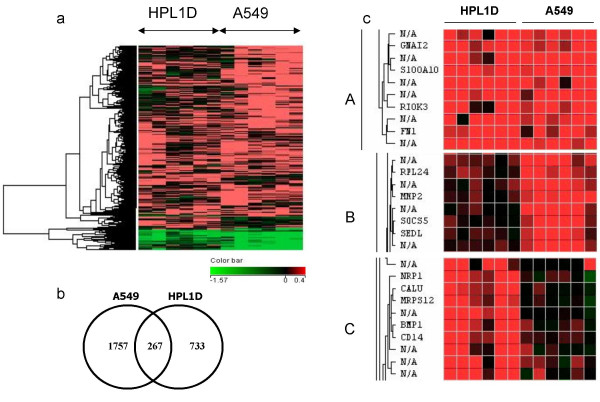
Comparison of genes regulated by TGF-β in HPL1D and A549 cells. a, Hierarchical clustering of genes regulated by TGF-β in HPL1D and A549 cells Genes regulated by TGF-β in HPL1D and A549 cells were clustered using Avadis 3.3 software. For this comparison only those genes for which expression values are available in both the cell-lines have been considered. The dendrogram on the left shows different clusters of genes segregated according to the pattern of regulation in the two cell-lines. b, Venn diagram showing the genes regulated by TGF-β either common or exclusively to the cell-lines and c, Expanded clusters showing sub-classes of genes regulated by TGF-β in HPL1D and A549 (derived from Figure 2a); A, Genes regulated by TGF-β in both HPL1D and A549 (267 genes); B, Genes regulated by TGF-β in A549 and not in HPL1D (1757 genes); C, Genes regulated by TGF-β in HPL1D and not in A549 (733 genes).
Validation of microarray results by qRT-PCR
The microarray data have been validated by real time and semi-quantitative RT-PCR. TMEPA, TGFBIP, IGFBP7, Integrin α V, TSP-1, MMP2 and TGM2 were studied by real time qRT-PCR as described in the methods. The primer sequences used for qRT-PCR are shown in table 1. Any gene is considered as regulated by TGF-β if qRT-PCR results showed > two fold regulation as compared to untreated cells. As shown in figure 3 and 4, TMEPA, TGFBIP and MMP2 are regulated by TGF-β 1 in both HPL1D and A549 cell-lines. In HPL1D, induction of TGFBIP and TMEPA by TGF-β is seen after 4 hours of treatment and increases with prolonged incubation. The induction of MMP2 by TGF-β is seen 6 hours onwards, whereas, there is no regulation of the genes IGFBP7, TSP-1, TGM2 after TGF-β treatment. Integrin αV, showed a 1.8 fold regulation after 24 hours of treatment with TGF-β. In A549, the induction of all these genes except TGM2 is observed after 4 hours of treatment and increase progressively with time, whereas, the induction of TGM2 increases till 12 hours and then declines. In addition, regulation of few other genes by TGF-β such as fibronectin 1, ZF36, S100A2, Tβ RI, ACTA2, T-plastin, keratin 7 and v-jun were assessed by semi-quantitative RT-PCR (See additional file 4, Fig. S1A). The data suggest that all these genes are regulated by 1.5 – 2.0 fold in HPL1D cells (See additional file 4, Fig. S1B). However, in A549 cells, the regulation of ZF36, ACTA2, Keratin 7, Tβ RI and v-jun is > 2.0 fold whereas, FN1, T-plastin and S100A2 is 1.5–2.0 fold (See additional file 4, Fig. S1C).
Table 1.
List of genes selected for validation by real-time RT-PCR
| Gene Name | Fold regulation in microarray | Primer sequences | |
| HPL1D cells | A549 cells | ||
| TGF-β induced protein 68 kDa (TGFBIP) | 1.8 | 2.8 | F 5' tgtgtgctgaagccatcgttg 3' R 5' ccggcttgtctgaaaaggtca 3' |
| Trans membrane prostate androgen induced RNA (TMEPA) | 1.5 | 3.08 | F5'ttcattccctgtcctcattgg3' R5'gcacaacagccatggaatca3' |
| Insulin like growth factor binding protein 7 (IGFBP7) | 1.49 | 2.72 | 5' ggtccttccatagtgacgcc 3' 5' tctgaatggccaggttgtcc 3' |
| Matrix metalloprotease 2 (MMP2) | 1.0 | 1.32 | 5' ctgatggcacccatttacacc 3' 5' gcctcgtataccgcatcaatc 3' |
| Transglutaminase 2 (TGM2) | 1.2 | 3.27 | 5' ccatgaccagaacagcaacct3' 5' tgacctccgcaaagacaaag 3' |
| Thrombospondin 1 (TSP-1) | 1.1 | 2.24 | 5' ccggcgtgaagtgtactagcta3' 5' tgcacttggcgttcttgtt 3' |
| Integrin αV | 1.1 | 2.73 | 5' caggcttgcaacccattct 3' 5' cctggcgagtttggttttct 3' |
| RPL35a | 5' gggtacagcatcactcgga 3' 5' acgcccgagatgaaacag 3' |
||
The genes were selected based on any or both of the following criteria. 1. Genes that showed differential regulation between cell-lines and show maximum regulation in at least one of the cell-lines, 2. Genes that showed regulation in the array and are known as TGF-β regulated genes in other studies. The primer sequences are shown 5' to 3' and F and R denote forward and reverse primers respectively.
Figure 3.
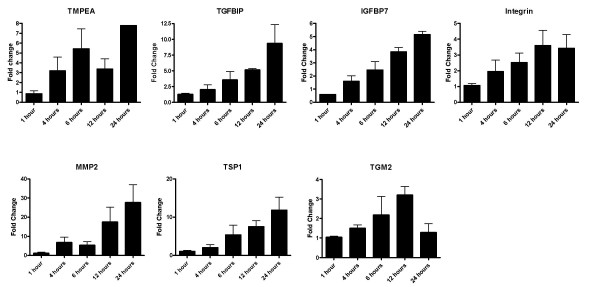
qRT-PCR analyses of selected genes (TMEPA, TGFBIP, IGFBP7, Integrin αV, TSP-1, MMP2, TGM2) with respect to TGF-β treatment in A549 cells. The cells were grown to 90% confluence, washed with serum free medium and treated with 5ng/ml TGF-β 1 for 1, 4, 6, 12 and 24 hours along with untreated controls at each time-point. Two microgram of total RNA from each treatment was reverse transcribed and cDNA equivalent to 10ng total RNA was used for the PCR reactions. The graphs represent the fold change over untreated controls after normalization with the expression of RPL35a. The bars show mean ± SD of two experiments of the PCR reactions done in duplicates.
Figure 4.
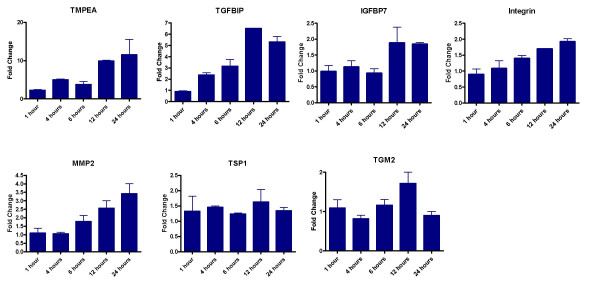
qRT-PCR analyses of selected genes (TMEPA, TGFBIP, IGFBP7, Integrin αV, TSP-1, MMP2, TGM2) with respect to TGF-β treatment in HPL1D cells. The cells were grown to 90% confluence, washed with serum free medium and treated with 5ng/ml TGF-β 1 for 1, 4, 6, 12 and 24 hours along with untreated controls at each time-point. Two microgram of total RNA from each treatment was reverse transcribed and cDNA equivalent to 10ng total RNA was used for the PCR reactions. The graphs represent the fold change over untreated controls after normalization with the expression of RPL35a. The bars show mean ± SD of two experiments of the PCR reactions done in duplicates.
Functional categories of genes regulated by TGF-β in HPL1D and A549
The lists of the regulated genes were fed into DAVID database (database for annotation, visualization and integrated discovery [17]. Based on the functions of the genes assigned by DAVID, genes were classified into the following categories. In HPL1D, TGF-β regulated genes belong to (a) regulation of actin cytoskeleton (table 2), (b) Focal adhesion (table 3) and (c) Wnt signalling pathways (table 4). In A549, TGF-β modulated genes belong to (a) MAP kinase signalling (table 5), (b) tight junction (table 6), (c) adherans junction (table 7) (d) focal adhesion (table 8) (e) insulin signalling (table 9) (f) regulation of actin cytoskeleton (table 10) and (g) Wnt signalling (table 11).
Table 2.
TGF-β regulated genes (17) in HPL1D involved in regulation of actin cytoskeleton
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| W20454 | FIBRONECTIN 1 | 1.23 ± 0 | 1.36 ± 0.04 | 2.1 ± 0.19 |
| H58824 | DIAPHANOUS HOMOLOG 1 (DROSOPHILA) | 0.84 ± 0.1 | 1.57 ± 0.19 | 1 ± 0.11 |
| N46724 | RHO GUANINE NUCLEOTIDE EXCHANGE FACTOR (GEF) 7 | 1.4 ± 0.07 | 0.96 ± 0.21 | 1 ± 0.07 |
| AA045207 | ACTIN RELATED PROTEIN 2/3 COMPLEX, SUBUNIT 5, 16KDA | 1.3 ± 0.09 | 1.3 ± 0.23 | 1.55 ± 0.12 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.11 ± 0.08 | 1.14 ± 0.03 | 1.55 ± 0.07 |
| BQ017489 | ACTININ, ALPHA 1 | 1.42 ± 0.03 | 1.02 ± 0.11 | 1.53 ± 0.2 |
| H73128 | INTEGRIN, BETA 5 | 1.06 ± 0.03 | 1.21 ± 0.03 | 1.47 ± 0.11 |
| W56632 | CD14 ANTIGEN | 1.46 ± 0 | 1.15 ± 0 | 1.45 ± 0.06 |
| AA046597 | INTEGRIN, BETA 4 | 1.63 ± 0.14 | 1.57 ± 0 | 1.43 ± 0.07 |
| H23109 | FIBROBLAST GROWTH FACTOR 1 (ACIDIC) | 1.55 ± 0.1 | 0.94 ± 0.08 | 1.42 ± 0.2 |
| AA010526 | GLUCOCORTICOID RECEPTOR DNA BINDING FACTOR 1 | 1.37 ± 0.26 | 1.29 ± 0.07 | 1.36 ± 0.02 |
| W67710 | PLATELET-DERIVED GROWTH FACTOR BETA POLYPEPTIDE (SIMIAN SARCOMA VIRAL (V-SIS) ONCOGENE HOMOLOG) | 1.53 ± 0.07 | 1.17 ± 0.02 | 1.2 ± 0.01 |
| AA037763 | WAS PROTEIN FAMILY, MEMBER 2 | 1.41 ± 0.08 | 1.08 ± 0.02 | 1.18 ± 0.05 |
| W39107 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.37 ± 0.05 | 1.07 ± 0.04 | 1.14 ± 0.16 |
| AL598940 | MYOSIN, LIGHT POLYPEPTIDE 1, ALKALI; SKELETAL, FAST | 1.36 ± 0.05 | 1.06 ± 0.12 | 1.07 ± 0.02 |
| AA131272 | INTEGRIN, ALPHA 8 | 1.19 ± 0 | 1.38 ± 0.06 | 0.96 ± 0.02 |
| H83405 | FYVE, RHOGEF AND PH DOMAIN CONTAINING 1 (FACIOGENITAL DYSPLASIA) | 0.45 ± 0.01 | 0.52 ± 0.17 | 0.25 ± 0 |
The list in the table shows the regulated genes as revealed by the DAVID tool. The columns show Genbank accession no., gene name, and fold change with respect to untreated controls at 1 hr, 4 hr and 12 hr time points following TGF-β treatment respectively. The regulation shown against each gene is depicted as fold change ± S.E. with respect to untreated cells. Each experimental value of the duplicates of the respective genes is shown in tables S1 and S3 (see additional file 1 and 3). The number in the parenthesis of the title represents the number of genes in the table.
Table 3.
TGF-β regulated genes (19) in HPL1D involved in focal adhesion
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| AA031376 | PLACENTAL GROWTH FACTOR, VASCULAR ENDOTHELIAL GROWTH FACTOR-RELATED PROTEIN | 1.18 ± 0.47 | 1.36 ± 0.05 | 1.16 ± 0.04 |
| AA046597 | INTEGRIN, BETA 4 | 1.63 ± 0.14 | 1.57 ± 0 | 1.43 ± 0.07 |
| AA131272 | INTEGRIN, ALPHA 8 | 1.19 ± 0 | 1.38 ± 0.06 | 0.96 ± 0.02 |
| AW959692 | VASCULAR ENDOTHELIAL GROWTH FACTOR C | 1.15 ± 0.17 | 1.34 ± 0.02 | 1.44 ± 0.31 |
| N74737 | COLLAGEN, TYPE IV, ALPHA 2 | 0.84 ± 0.01 | 1 ± 0.04 | 1.51 ± 0.01 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.3 ± 0 | 1.14 ± 0.03 | 1.1 ± 0.07 |
| AL079948 | THROMBOSPONDIN 1 | 0.78 ± | 1.82 ± 0.09 | 2.5 ± 0.1 |
| H73128 | INTEGRIN, BETA 5 | 1.06 ± 0.03 | 1.21 ± 0.03 | 1.47 ± 0.11 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.11 ± 0.08 | 1.14 ± 0.03 | 1.55 ± 0.07 |
| BM801832 | CAVEOLIN 1, CAVEOLAE PROTEIN, 22 KDA | 1.09 ± 0.11 | 1.4 ± 0.01 | 1.47 ± 0 |
| W67710 | PLATELET-DERIVED GROWTH FACTOR BETA POLYPEPTIDE (SIMIAN SARCOMA VIRAL (V-SIS) ONCOGENE HOMOLOG) | 1.53 ± 0.07 | 1.17 ± 0.02 | 1.2 ± 0.01 |
| W20454 | FIBRONECTIN 1 | 1.23 ± 0 | 1.36 ± 0.04 | 2.1 ± 0.19 |
| AA029359 | LAMININ, BETA 2 (LAMININ S) | 1.54 ± 0.06 | 1.17 ± 0.08 | 1.02 ± 0.06 |
| W39107 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.37 ± 0.05 | 1.07 ± 0.04 | 1.14 ± 0.16 |
| AA043301 | COLLAGEN, TYPE IV, ALPHA 1 | 1.4 ± 0.19 | 1.52 ± | 1.52 ± 0.03 |
| BQ017489 | ACTININ, ALPHA 1 | 1.42 ± 0.03 | 1.02 ± 0.11 | 1.53 ± 0.2 |
| AA010526 | GLUCOCORTICOID RECEPTOR DNA BINDING FACTOR 1 | 1.37 ± 0.26 | 1.29 ± 0.07 | 1.36 ± 0.02 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.34 ± 0.02 | 0.81 ± 0.1 | 0.94 ± 0.03 |
| H58824 | DIAPHANOUS HOMOLOG 1 (DROSOPHILA) | 0.84 ± 0.1 | 1.57 ± 0.19 | 1 ± 0.11 |
For description see legend of table 2
Table 4.
TGF-β regulated genes (15) in HPL1D involved in wnt signaling
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| N92026 | PROTEIN PHOSPHATASE 3 (FORMERLY 2B), CATALYTIC SUBUNIT, BETA ISOFORM (CALCINEURIN A BETA) | 0.92 ± 0.06 | 1.31 ± 0.01 | 1.05 ± 0.11 |
| H82025 | PRICKLE-LIKE 1 (DROSOPHILA) | 1.79 ± 0.18 | 1.17 ± 0.02 | 1.21 ± |
| AA044665 | F-BOX AND WD-40 DOMAIN PROTEIN 11 | 1.24 ± 0.18 | 1.14 ± | 1.42 ± 0.08 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.3 ± 0 | 1.14 ± 0.03 | 1.1 ± 0.07 |
| AA203216 | PROTEIN PHOSPHATASE 2 (FORMERLY 2A), REGULATORY SUBUNIT B (PR 52), BETA ISOFORM | 1.26 ± 0 | 1.07 ± 0.2 | 1.5 ± 0.04 |
| H79065 | PRICKLE-LIKE 2 (DROSOPHILA) | 1.38 ± 0.07 | 1.04 ± 0.28 | 1.01 ± 0.14 |
| BM921730 | PROTEIN PHOSPHATASE 2 (FORMERLY 2A), REGULATORY SUBUNIT A (PR 65), BETA ISOFORM | 1.48 ± 0.01 | 1.19 ± 0.06 | 1.17 ± 0.01 |
| R23027 | CASEIN KINASE 1, ALPHA 1 | 1.28 ± 0.08 | 1.46 ± 0.01 | 1.26 ± 0.08 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.11 ± 0.08 | 1.14 ± 0.03 | 1.55 ± 0.07 |
| BM311217 | WINGLESS-TYPE MMTV INTEGRATION SITE FAMILY, MEMBER 4 | 1.26 ± 0.09 | 1 ± 0.06 | 1.34 ± 0.02 |
| W86518 | C-TERMINAL BINDING PROTEIN 2 | 1.33 ± 0.01 | 1.35 ± 0.24 | 1.13 ± 0.07 |
| H70045 | CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE (CAM KINASE) II GAMMA | 1.48 ± 0.16 | 1.1 ± 0.01 | 1.16 ± 0.05 |
| W39107 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.37 ± 0.05 | 1.07 ± 0.04 | 1.14 ± 0.16 |
| BE618078 | CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE (CAM KINASE) II BETA | 1.58 ± 0.02 | 0.86 ± 0.05 | 1.02 ± 0.1 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.34 ± 0.02 | 0.81 ± 0.1 | 0.94 ± 0.03 |
For description see legend of table 2
Table 5.
TGF-β regulated genes (38) in A549 involved in MAP kinase signalling
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| H09832 | ACTIVIN A RECEPTOR, TYPE IB | 1.01 ± 0.17 | 1.66 ± 0.29 | 1.17 ± 0.06 |
| N27681 | HEAT SHOCK 70 KDA PROTEIN 1A | 1.12 ± 0.01 | 1.46 ± 0.18 | 1.42 ± 0.08 |
| H73255 | MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE 6 | 0.84 ± 0.31 | 1.04 ± 0.07 | 1.49 ± 0.11 |
| N42722 | GUANINE NUCLEOTIDE BINDING PROTEIN (G PROTEIN), GAMMA 12 | 1.44 ± 0.04 | 1.5 ± 0.09 | 1.28 ± 0.17 |
| AA039598 | RIBOSOMAL PROTEIN S6 KINASE, 90 KDA, POLYPEPTIDE 1 | 1.16 ± 0.13 | 1.21 ± 0.02 | 1.32 ± 0.02 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.33 ± 0.23 | 1.56 ± 0.08 | 1.36 ± 0.03 |
| H73472 | CELL DIVISION CYCLE 42 (GTP BINDING PROTEIN, 25 KDA) | 1.24 ± 0.27 | 1.33 ± 0.07 | 1.56 ± 0.01 |
| H62639 | HEAT SHOCK 70 KDA PROTEIN 8 | 1.29 ± 0.01 | 1.68 ± 0.18 | 1.37 ± 0 |
| H23019 | TRANSFORMING GROWTH FACTOR, BETA RECEPTOR I (ACTIVIN A RECEPTOR TYPE II-LIKE KINASE, 53 KDA) | 1.09 ± 0.01 | 2.53 ± 0.07 | 2.11 ± 0.41 |
| AA088648 | FIBROBLAST GROWTH FACTOR RECEPTOR 1 (FMS-RELATED TYROSINE KINASE 2, PFEIFFER SYNDROME) | 1.45 ± 0.24 | 1.97 ± 0.08 | 1.77 ± 0.09 |
| AV747676 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 3 (PROTEIN KINASE B, GAMMA) | 1.06 ± 0.13 | 1.46 ± 0.05 | 1.18 ± 0.01 |
| AI500475 | RAS GUANYL RELEASING PROTEIN 1 (CALCIUM AND DAG-REGULATED) | 1.31 ± 0.05 | 1.37 ± 0 | 1.41 ± 0.07 |
| R86053 | NUCLEAR FACTOR OF KAPPA LIGHT POLYPEPTIDE GENE ENHANCER IN B-CELLS 2 (P49/P100) | 0.99 ± 0.12 | 0.9 ± 0.07 | 1.44 ± 0.09 |
| BE502112 | RIBOSOMAL PROTEIN S6 KINASE, 90 KDA, POLYPEPTIDE 3 | 0.37 ± 0.1 | 0.29 ± 0.05 | 0.41 ± 0.06 |
| H23109 | FIBROBLAST GROWTH FACTOR 1 (ACIDIC) | 0.94 ± | 1.36 ± 0.01 | 1.13 ± 0.05 |
| AA127934 | PROTEIN KINASE, X-LINKED | 1.21 ± 0.07 | 1.38 ± 0.01 | 1.35 ± 0.07 |
| H89206 | PROTEIN KINASE, CAMP-DEPENDENT, CATALYTIC, BETA | 1.18 ± 0.08 | 1.42 ± 0.04 | 1.25 ± 0.03 |
| R14348 | MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE 5 | 1.45 ± 0.09 | 1.22 ± 0.17 | 1.03 ± 0.06 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.39 ± 0 | 1.41 ± 0.06 | 1.28 ± 0.18 |
| H11708 | MITOGEN-ACTIVATED PROTEIN KINASE KINASE 4 | 1.38 ± 0.02 | 1.54 ± 0.1 | 1.24 ± 0.17 |
| R36401 | FILAMIN B, BETA (ACTIN BINDING PROTEIN 278) | 0.96 ± 0.02 | 1.17 ± 0.11 | 1.33 ± 0.01 |
| BI758537 | MADS BOX TRANSCRIPTION ENHANCER FACTOR 2, POLYPEPTIDE C (MYOCYTE ENHANCER FACTOR 2C) | 1.77 ± 0.42 | 1.25 ± | 1.36 ± 0.09 |
| W56249 | ACTIVATING TRANSCRIPTION FACTOR 2 | 1.25 ± 0.13 | 1.5 ± 0.02 | 1.72 ± 0.18 |
| AA034981 | INTERLEUKIN 1 RECEPTOR, TYPE I | 0.37 ± 0.08 | 0.4 ± 0.02 | 0.29 ± 0.04 |
| AA056664 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 1 | 1.34 ± 0.15 | 1.44 ± 0.05 | 1.36 ± 0.07 |
| H20677 | MITOGEN-ACTIVATED PROTEIN KINASE KINASE KINASE 7 INTERACTING PROTEIN 1 | 1.13 ± 0.15 | 0.96 ± 0.1 | 1.6 ± 0.01 |
| BM999610 | V-JUN SARCOMA VIRUS 17 ONCOGENE HOMOLOG (AVIAN) | 1.57 ± 0.26 | 2.13 ± 0.01 | 1.66 ± 0.18 |
| BE781203 | CALCIUM CHANNEL, VOLTAGE-DEPENDENT, ALPHA 1G SUBUNIT | 1.13 ± 0.03 | 1.02 ± 0 | 1.44 ± 0 |
| T83272 | RAS P21 PROTEIN ACTIVATOR 2 | 0.96 ± 0.08 | 1.12 ± 0.18 | 1.33 ± 0.02 |
| T75436 | MITOGEN-ACTIVATED PROTEIN KINASE 10 | 1.16 ± 0.17 | 1.22 ± 0.05 | 1.37 ± 0.07 |
| W70006 | CELL DIVISION CYCLE 25B | 1.47 ± 0.1 | 1.15 ± 0.05 | 1.38 ± 0.08 |
| R32409 | PHOSPHOLIPASE A2, GROUP V | 1.14 ± 0.27 | 1.27 ± 0.01 | 1.5 ± 0.07 |
| AW961873 | CALCIUM CHANNEL, VOLTAGE-DEPENDENT, GAMMA SUBUNIT 4 | 1.45 ± 0.15 | 1.23 ± 0.17 | 1.02 ± 0 |
| BI752921 | PLATELET-DERIVED GROWTH FACTOR RECEPTOR, BETA POLYPEPTIDE | 1.43 ± 0.09 | 1.03 ± 0.02 | 1.09 ± 0.07 |
| BQ055308 | RIBOSOMAL PROTEIN L4 | 1.44 ± 0.05 | 1.26 ± 0.04 | 1.43 ± 0.08 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.54 ± 0.06 | 1.08 ± 0.01 | 1.15 ± 0 |
| BQ016117 | V-FOS FBJ MURINE OSTEOSARCOMA VIRAL ONCOGENE HOMOLOG | 1.48 ± 0.26 | 1.44 ± 0.04 | 1.29 ± 0.06 |
| W05234 | CALCIUM CHANNEL, VOLTAGE-DEPENDENT, BETA 3 SUBUNIT | 1.4 ± 0.07 | 1.15 ± 0.05 | 1.27 ± 0 |
The list in the table shows the regulated genes as revealed by the DAVID tool. The columns show Genbank accession no., gene name, and fold change with respect to untreated controls at 1 hr, 4 hr and 12 hr time points following TGF-β treatment respectively. The regulation shown against each gene is depicted as fold change ± S.E. with respect to untreated cells. Each experimental value of the duplicates of respective genes is shown in tables S1 and S2 (see additional files 1 and 2). The number in the parenthesis of the title represents the number of genes in the table.
Table 6.
TGF-β regulated genes (21) in A549 involved in tight junction
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| AA027986 | PROTEIN PHOSPHATASE 2 (FORMERLY 2A), CATALYTIC SUBUNIT, ALPHA ISOFORM | 1.24 ± 0.15 | 1.65 ± 0.03 | 1.34 ± 0.11 |
| H80401 | V-YES-1 YAMAGUCHI SARCOMA VIRAL ONCOGENE HOMOLOG 1 | 0.3 ± 0.19 | 0.2 ± 0.07 | 0.29 ± 0.01 |
| R52046 | ACTININ, ALPHA 4 | 1.26 ± 0.68 | 1.21 ± | 1.81 ± 0.21 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.33 ± 0.23 | 1.56 ± 0.08 | 1.36 ± 0.03 |
| BE738680 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.24 ± 0.11 | 1.4 ± 0.06 | 1.51 ± 0.03 |
| H73472 | CELL DIVISION CYCLE 42 (GTP BINDING PROTEIN, 25 KDA) | 1.24 ± 0.27 | 1.33 ± 0.07 | 1.56 ± 0.01 |
| N92643 | CALCIUM/CALMODULIN-DEPENDENT SERINE PROTEIN KINASE (MAGUK FAMILY) | 1.31 ± | 1.45 ± 0.01 | 1.03 ± 0.01 |
| BI828016 | SPECTRIN, ALPHA, NON-ERYTHROCYTIC 1 (ALPHA-FODRIN) | 1.33 ± 0.08 | 1.38 ± 0.01 | 1.93 ± 0.04 |
| AV747676 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 3 (PROTEIN KINASE B, GAMMA) | 1.06 ± 0.13 | 1.46 ± 0.05 | 1.18 ± 0.01 |
| AA031382 | GUANINE NUCLEOTIDE BINDING PROTEIN (G PROTEIN), ALPHA INHIBITING ACTIVITY POLYPEPTIDE 2 | 1.23 ± 0.04 | 1.21 ± 0.05 | 1.65 ± 0.21 |
| R14061 | PAR-3 PARTITIONING DEFECTIVE 3 HOMOLOG (C. ELEGANS) | 1.52 ± 0.07 | 1.12 ± 0.04 | 1.05 ± 0 |
| AA056664 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 1 | 1.34 ± 0.15 | 1.44 ± 0.05 | 1.36 ± 0.07 |
| BI553762 | CATENIN (CADHERIN-ASSOCIATED PROTEIN), ALPHA 2 | 1.36 ± 0.06 | 1.17 ± 0.03 | 1.88 ± 0.21 |
| BM993684 | CORTACTIN | 1.63 ± 0.07 | 1.32 ± 0.11 | 1.26 ± 0.08 |
| AA009869 | ERYTHROCYTE MEMBRANE PROTEIN BAND 4.1 (ELLIPTOCYTOSIS 1, RH-LINKED) | 1.19 ± 0.13 | 1.21 ± 0.05 | 1.39 ± 0.02 |
| AA058385 | TIGHT JUNCTION PROTEIN 2 (ZONA OCCLUDENS 2) | 1.35 ± 0.15 | 1.36 ± 0.02 | 1.16 ± 0.18 |
| AI375078 | KIAA1634 PROTEIN | 1.48 ± 0.02 | 1.25 ± 0.03 | 1.04 ± 0.01 |
| BM978991 | MEMBRANE ASSOCIATED GUANYLATE KINASE, WW AND PDZ DOMAIN CONTAINING 2 | 1.1 ± 0.05 | 1.15 ± 0 | 1.47 ± 0.05 |
| N43838 | ERYTHROCYTE MEMBRANE PROTEIN BAND 4.1-LIKE 3 | 1.4 ± 0.08 | 1.15 ± 0.14 | 1.15 ± 0.09 |
| BQ017489 | ACTININ, ALPHA 1 | 1.57 ± 0.22 | 1.24 ± 0.1 | 1.5 ± 0.1 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.54 ± 0.06 | 1.08 ± 0.01 | 1.15 ± 0 |
For description see legend of table 5
Table 7.
TGF-β regulated genes (21) in A549 involved in adherans junction
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| H78804 | SMAD, MOTHERS AGAINST DPP HOMOLOG 3 (DROSOPHILA) | 1.22 ± 0.09 | 1.36 ± 0.02 | 1.15 ± 0.06 |
| H09832 | ACTIVIN A RECEPTOR, TYPE IB | 1.01 ± 0.17 | 1.66 ± 0.29 | 1.17 ± 0.06 |
| H80401 | V-YES-1 YAMAGUCHI SARCOMA VIRAL ONCOGENE HOMOLOG 1 | 0.3 ± 0.19 | 0.2 ± 0.07 | 0.29 ± 0.01 |
| R52046 | ACTININ, ALPHA 4 | 1.26 ± 0.68 | 1.21 ± | 1.81 ± 0.21 |
| AA029516 | TRANSCRIPTION FACTOR 7-LIKE 2 (T-CELL SPECIFIC, HMG-BOX) | 1.25 ± 0.02 | 1.41 ± 0.02 | 1.14 ± 0.2 |
| BE738680 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.24 ± 0.11 | 1.4 ± 0.06 | 1.51 ± 0.03 |
| H73472 | CELL DIVISION CYCLE 42 (GTP BINDING PROTEIN, 25 KDA) | 1.24 ± 0.27 | 1.33 ± 0.07 | 1.56 ± 0.01 |
| H23019 | TRANSFORMING GROWTH FACTOR, BETA RECEPTOR I (ACTIVIN A RECEPTOR TYPE II-LIKE KINASE, 53 KDA) | 1.09 ± 0.01 | 2.53 ± 0.07 | 2.11 ± 0.41 |
| AA088648 | FIBROBLAST GROWTH FACTOR RECEPTOR 1 (FMS-RELATED TYROSINE KINASE 2, PFEIFFER SYNDROME) | 1.45 ± 0.24 | 1.97 ± 0.08 | 1.77 ± 0.09 |
| R55134 | TRANSCRIPTION FACTOR 7-LIKE 1 (T-CELL SPECIFIC, HMG-BOX) | 1.45 ± 0.03 | 1.2 ± 0.1 | 1.27 ± 0.05 |
| BM314539 | CATENIN (CADHERIN-ASSOCIATED PROTEIN), DELTA 1 | 1.35 ± 0.04 | 1.21 ± 0.08 | 1.05 ± 0.06 |
| R14061 | PAR-3 PARTITIONING DEFECTIVE 3 HOMOLOG (C. ELEGANS) | 1.52 ± 0.07 | 1.12 ± 0.04 | 1.05 ± 0 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.39 ± 0 | 1.41 ± 0.06 | 1.28 ± 0.18 |
| W24540 | FERM, RHOGEF AND PLECKSTRIN DOMAIN PROTEIN 2 | 1.28 ± 0.06 | 1.37 ± 0.1 | 1.46 ± 0.05 |
| BI553762 | CATENIN (CADHERIN-ASSOCIATED PROTEIN), ALPHA 2 | 1.36 ± 0.06 | 1.17 ± 0.03 | 1.88 ± 0.21 |
| R35167 | BAI1-ASSOCIATED PROTEIN 2 | 1.46 ± 0.15 | 0.97 ± 0.02 | 1.32 ± 0.12 |
| T82805 | PROTEIN TYROSINE PHOSPHATASE, RECEPTOR TYPE, J | 1.49 ± 0.09 | 1.35 ± 0.13 | 1.19 ± 0.08 |
| AA069424 | INSULIN-LIKE GROWTH FACTOR 1 RECEPTOR | 1.5 ± 0.09 | 1.16 ± 0.07 | 1.02 ± 0.05 |
| N77112 | IQ MOTIF CONTAINING GTPASE ACTIVATING PROTEIN 1 | 1.6 ± 0.35 | 1.54 ± 0.13 | 1.48 ± 0.04 |
| BQ017489 | ACTININ, ALPHA 1 | 1.57 ± 0.22 | 1.24 ± 0.1 | 1.5 ± 0.1 |
| AA005247 | MET PROTO-ONCOGENE (HEPATOCYTE GROWTH FACTOR RECEPTOR) | 1.68 ± 0.34 | 1.3 ± 0.03 | 1.14 ± 0.07 |
For description see legend of table 5
Table 8.
TGF-β regulated genes (36) in A549 involved in focal adhesion
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| N45505 | VAV 1 ONCOGENE | 0.64 ± 0.37 | 0.41 ± 0.11 | 0.31 ± 0.01 |
| AA046597 | INTEGRIN, BETA 4 | 1.26 ± 0.21 | 1.1 ± 0.19 | 1.8 ± 0.18 |
| R52046 | ACTININ, ALPHA 4 | 1.26 ± 0.68 | 1.21 ± | 1.81 ± 0.21 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.33 ± 0.23 | 1.56 ± 0.08 | 1.36 ± 0.03 |
| BE738680 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.24 ± 0.11 | 1.4 ± 0.06 | 1.51 ± 0.03 |
| H73472 | CELL DIVISION CYCLE 42 (GTP BINDING PROTE IN, 25 KDA) | 1.24 ± 0.27 | 1.33 ± 0.07 | 1.56 ± 0.01 |
| W17002 | INTEGRIN, ALPHA V (VITRONECTIN RECEPTOR, ALPHA POLYPEPTIDE, ANTIGEN CD51) | 1.21 ± 0.06 | 2.32 ± 0.29 | 2.73 ± 0.28 |
| AV747676 | V-AKTMURINE THYMOMA VIRAL ONCOGENE HOMOLOG 3 (PROTEIN KINASE B, GAMMA) | 1.06 ± 0.13 | 1.46 ± 0.05 | 1.18 ± 0.01 |
| BM912016 | P21(CDKN1A)-ACTIVATED KINASE 4 | 1.29 ± 0.03 | 1.1 ± 0.23 | 1.54 ± 0.03 |
| BM008467 | ZYXIN | 0.95 ± 0.1 | 1.16 ± 0.14 | 1.41 ± 0.07 |
| AA037738 | HEPATOCYTE GROWTH FACTOR (HEPAPOIETIN A; SCATTER FACTOR) | 1.43 ± 0.07 | 1.36 ± 0.03 | 1.06 ± 0.13 |
| H89256 | LAMININ, BETA 1 | 1.44 ± 0.02 | 1.4 ± 0.02 | 1.12 ± 0.07 |
| AA026831 | KINASE INSERT DOMAIN RECEPTOR (A TYPE III RECEPTOR TYROSINE KINASE) | 1.17 ± 0.1 | 1.34 ± 0.02 | 1.06 ± 0.01 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.39 ± 0 | 1.41 ± 0.06 | 1.28 ± 0.18 |
| AA122291 | COLLAGEN, TYPE V, ALPHA 2 | 1.1 ± 0.08 | 1.19 ± 0.13 | 1.43 ± 0.11 |
| R36401 | FILAMIN B, BETA (ACTIN BINDING PROTEIN 278) | 0.96 ± 0.02 | 1.17 ± 0.11 | 1.33 ± 0.01 |
| R20452 | CALPAIN 10 | 1.19 ± 0.21 | 1.08 ± 0.06 | 1.5 ± 0.01 |
| H15287 | P21(CDKN1A)-ACTIVATED KINASE 6 | 1.47 ± 0.08 | 1.21 ± 0.05 | 1.57 ± 0.04 |
| W24540 | FERM, RHOGEF AND PLECKSTRIN DOMAIN PROTEIN 2 | 1.28 ± 0.06 | 1.37 ± 0.1 | 1.46 ± 0.05 |
| T39460 | PROTEIN PHOSPHATASE 1, CATALYTIC SUBUNIT, ALPHA ISOFORM | 1.35 ± 0.17 | 1.38 ± 0.04 | 1.49 ± 0.15 |
| AA056664 | V-AKT MURINE THYMOMA VTRAL ONCOGENE HOMOLOG 1 | 1.34 ± 0.15 | 1.44 ± 0.05 | 1.36 ± 0.07 |
| AA127854 | INTEGRIN, ALPHA 5 (FIBRONECTIN RECEPTOR, ALPHA POLYPEPTIDE) | 1.4 ± 0.08 | 1.12 ± 0.03 | 1.22 ± 0.06 |
| BG497332 | THROMBOSPONDIN 1 | 0.87 ± 0.01 | 1.1 ± 0.1 | 2.24 ± 0.11 |
| R25275 | GLYCOGEN SYNTHASE KINASE 3 BETA. | 1.15 ± 0.16 | 1.36 ± 0.04 | 1.23 ± 0.06 |
| BQ016959 | COLLAGEN, TYPE IV, ALPHA 2 | 1.18 ± 0.07 | 1.3 ± 0 | 1.33 ± 0.09 |
| BM999610 | V-JUN SARCOMA VIRUS 17 ONCOGENE HOMOLOG (AVIAN) | 1.57 ± 0.26 | 2.13 ± 0.01 | 1.66 ± 0.18 |
| AA044582 | INTEGRIN, BETA 8 | 0.33 ± 0.03 | 0.26 ± 0.04 | 0.25 ± 0 |
| T75436 | MITOGEN-ACTIVATED PROTEIN KINASE 10 | 1.16 ± 0.17 | 1.22 ± 0.05 | 1.37 ± 0.07 |
| AA069424 | INSULIN-LIKE GROWTH FACTOR 1 RECEPTOR | 1.5 ± 0.09 | 1.16 ± 0.07 | 1.02 ± 0.05 |
| BI752921 | PLATELET-DERIVED GROWTH FACTOR RECEPTOR, BETA POLYPEPTIDE | 1.43 ± 0.09 | 1.03 ± 0.02 | 1.09 ± 0.07 |
| N99223 | INTEGRIN, ALPHA 9 | 1.46 ± 0.12 | 1.08 ± | 1.29 ± 0.01 |
| BQ017489 | ACTININ, ALPHA 1 | 1.57 ± 0.22 | 1.24 ± 0.1 | 1.5 ± 0.1 |
| AA010526 | GLUCOCORTICOID RECEPTOR DNA BINDING FACTOR 1 | 1.44 ± 0.14 | 1.03 ± 0.04 | 1.31 ± 0 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.54 ± 0.06 | 1.08 ± 0.01 | 1.15 ± 0 |
| AA005247 | MET PROTO-ONCOGENE (HEPATOCYTE GROWTH FACTOR RECEPTOR) | 1.68 ± 0.34 | 1.3 ± 0.03 | 1.14 ± 0.07 |
| AA043909 | PTK2 PROTEIN TYROSINE KINASE 2 | 0.52 ± 0.11 | 0.52 ± 0.12 | 0.34 ± 0 |
For description see legend of table 5
Table 9.
TGF-β regulated genes (26) in A549 involved in insulin signalling
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| R23436 | PROTEIN KINASE, CAMP-DEPENDENT, REGULATORY, TYPE II, ALPHA | 0.32 ± 0.03 | 0.52 ± 0.03 | 0.22 ± 0.01 |
| BI855956 | LIPASE, HORMONE-SENSITIVE | 1.17 ± 0.06 | 1.12 ± 0.1 | 1.38 ± 0.07 |
| W32908 | FORKHEAD BOX O1A (RHABDOMYOSARCOMA) | 0.46 ± 0.17 | 0.38 ± 0.01 | 0.3 ± 0.05 |
| R48236 | SUPPRESSOR OF CYTOKINE SIGNALING 3 | 1.02 ± 0.21 | 1.51 ± 0 | 1.45 ± 0.09 |
| BF034127 | INSULIN RECEPTOR SUBSTRATE 2 | 0.3 ± 0.2 | 0.2 ± 0.09 | 0.33 ± 0.01 |
| BG502582 | CALMODULIN 1 (PHOSPHORYLASE KINASE, DELTA) | 0.89 ± 0.02 | 1.41 ± 0.07 | 1.21 ± 0.14 |
| R74161 | PHOSPHORYLASE, GLYCOGEN; LIVER (HERS DISEASE, GLYCOGEN STORAGE DISEASE TYPE VI) | 0.23 ± 0.06 | 0.28 ± 0.08 | 0.28 ± 0.02 |
| AA030048 | PROTEIN KINASE, CAMP-DEPENDENT, REGULATORY, TYPE I, BETA | 1.1 ± 0.03 | 1.02 ± 0.06 | 1.31 ± 0.01 |
| BM556894 | RAPTOR | 0.89 ± | 1.34 ± 0.04 | 1.29 ± 0.19 |
| AV747676 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 3 (PROTEIN KINASE B, GAMMA) | 1.06 ± 0.13 | 1.46 ± 0.05 | 1.18 ± 0.01 |
| AA127934 | PROTEIN KINASE, X-LINKED | 1.21 ± 0.07 | 1.38 ± 0.01 | 1.35 ± 0.07 |
| H89206 | PROTEIN KINASE, CAMP-DEPENDENT, CATALYTIC, BETA | 1.18 ± 0.08 | 1.42 ± 0.04 | 1.25 ± 0.03 |
| W35243 | PHOSPHOFRUCTOKINASE, PLATELET | 1.15 ± 0.05 | 1.39 ± 0.06 | 1.34 ± 0.17 |
| T93745 | EUKARYOTIC TRANSLATION INITIATION FACTOR 4E MEMBER 2 | 1.4 ± 0 | 1.09 ± 0.11 | 1.06 ± 0.03 |
| T39460 | PROTEIN PHOSPHATASE 1, CATALYTIC SUBUNIT, ALPHA ISOFORM | 1.35 ± 0.17 | 1.38 ± 0.04 | 1.49 ± 0.15 |
| H80740 | PHOSPHOFRUCTOKINASE, MUSCLE | 1.42 ± 0.05 | 1.25 ± 0.04 | 1.36 ± 0.14 |
| AA056664 | V-AKT MURINE THYMOMA VIRAL ONCOGENE HOMOLOG 1 | 1.34 ± 0.15 | 1.44 ± 0.05 | 1.36 ± 0.07 |
| H52478 | TUBEROUS SCLEROSIS 2 | 1.23 ± 0.2 | 1.32 ± 0.05 | 1.4 ± 0.01 |
| R25275 | GLYCOGEN SYNTHASE KINASE 3 BETA | 1.15 ± 0.16 | 1.36 ± 0.04 | 1.23 ± 0.06 |
| H64260 | PROTEIN KINASE, AMP-ACTIVATED, GAMMA 2 NON-CATALYTIC SUBUNIT | 1.69 ± | 1.21 ± | 1.46 ± 0.05 |
| T75436 | MITOGEN-ACTIVATED PROTEIN KINASE 10 | 1.16 ± 0.17 | 1.22 ± 0.05 | 1.37 ± 0.07 |
| T75267 | FK506 BINDING PROTEIN 12-RAPAMYCIN ASSOCIATED PROTEIN 1 | 1.77 ± 0.24 | 1.37 ± 0.05 | 1.2 ± 0.06 |
| H44888 | SUPPRESSOR OF CYTOKINE SIGNALING 6 | 0.25 ± 0.13 | 0.16 ± | 0.18 ± 0.01 |
| H59587 | ACETYL-COENZYME A CARBOXYLASE ALPHA | 1.42 ± 0.44 | 1.32 ± 0.01 | 1.18 ± 0 |
| H08429 | TUBEROUS SCLEROSIS 1 | 1.43 ± 0.09 | 1.09 ± 0.07 | 1.35 ± 0.1 |
| AA010882 | GLYCOGEN SYNTHASE 1 (MUSCLE) | 1.46 ± 0.07 | 1.15 ± 0 | 1.01 ± 0.08 |
For description see legend of table 5
Table 10.
TGF-β regulated genes (36) in A549 involved in regulation of actin cytoskeleton
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| H83405 | FYVE, RHOGEF AND PH DOMAIN CONTAINING 1 (FACIOGENITAL DYSPLASIA) | 0.57 ± 0.41 | 0.35 ± | 0.26 ± 0.01 |
| N45505 | VAV 1 ONCOGENE | 0.64 ± 0.37 | 0.41 ± 0.11 | 0.31 ± 0.01 |
| N45518 | NCK-ASSOCIATED PROTEIN 1 | 1.03 ± 0.12 | 1.76 ± 0.2 | 1.39 ± 0.18 |
| AA046597 | INTEGRIN, BETA 4 | 1.26 ± 0.21 | 1.1 ± 0.19 | 1.8 ± 0.18 |
| R52046 | ACTININ, ALPHA 4 | 1.26 ± 0.68 | 1.21 ± | 1.81 ± 0.21 |
| N42722 | GUANINE NUCLEOTIDE BINDING PROTEIN (G PROTEIN), GAMMA 12 | 1.44 ± 0.04 | 1.5 ± 0.09 | 1.28 ± 0.17 |
| AL598940 | MYOSIN, LIGHT POLYPEPTIDE 1, ALKALI; SKELETAL, FAST | 1.15 ± 0.01 | 1.33 ± 0.17 | 1.56 ± 0.02 |
| BE738680 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.24 ± 0.11 | 1.4 ± 0.06 | 1.51 ± 0.03 |
| H73472 | CELL DIVISION CYCLE 42 (GTP BINDING PROTEIN, 25KDA) | 1.24 ± 0.27 | 1.33 ± 0.07 | 1.56 ± 0.01 |
| W17002 | INTEGRIN, ALPHA V (VITRONECTIN RECEPTOR, ALPHA POLYPEPTIDE, ANTIGEN CD51) | 1.21 ± 0.06 | 2.32 ± 0.29 | 2.73 ± 0.28 |
| AA088648 | FIBROBLAST GROWTH FACTOR RECEPTOR 1 (FMS-RELATED TYROSINE KINASE 2, PFEIFFER SYNDROME) | 1.45 ± 0.24 | 1.97 ± 0.08 | 1.77 ± 0.09 |
| N57424 | T-CELL LYMPHOMA INVASION AND METASTASIS 1 | 1.35 ± 0.02 | 1.23 ± 0.04 | 1.34 ± 0.02 |
| H10616 | PROFILIN 2 | 1.44 ± 0.08 | 1.49 ± 0.11 | 1.1 ± 0 |
| BM912016 | P21(CDKN1A)-ACTIVATED KINASE 4 | 1.29 ± 0.03 | 1.1 ± 0.23 | 1.54 ± 0.03 |
| H23109 | FIBROBLAST GROWTH FACTOR 1 (ACIDIC) | 0.94 ± | 1.36 ± 0.01 | 1.13 ± 0.05 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.39 ± 0 | 1.41 ± 0.06 | 1.28 ± 0.18 |
| H15287 | P21(CDKN1A)-ACTIVATED KINASE 6 | 1.47 ± 0.08 | 1.21 ± 0.05 | 1.57 ± 0.04 |
| T39460 | PROTEIN PHOSPHATASE 1, CATALYTIC SUBUNIT, ALPHA ISOFORM | 1.35 ± 0.17 | 1.38 ± 0.04 | 1.49 ± 0.15 |
| BM888157 | THYMOSIN, BETA 4, X-LINKED | 1.02 ± | 1.58 ± | 1.93 ± 0.14 |
| T83174 | ACTIN RELATED PROTEIN 2/3 COMPLEX, SUBUNIT 1B, 41KDA | 1.24 ± 0.08 | 1.06 ± 0.1 | 1.54 ± 0.04 |
| AA127854 | INTEGRIN, ALPHA 5 (FIBRONECTIN RECEPTOR, ALPHA POLYPEPTIDE) | 1.4 ± 0.08 | 1.12 ± 0.03 | 1.22 ± 0.06 |
| H27657 | G PROTEIN-COUPLED RECEPTOR KINASE INTERACTOR 1 | 1.39 ± 0.09 | 1.11 ± 0.04 | 1.34 ± 0.25 |
| N29131 | MOESIN | 1.37 ± 0.09 | 1.03 ± 0.06 | 1.42 ± 0.05 |
| AA044582 | INTEGRIN, BETA 8 | 0.33 ± 0.03 | 0.26 ± 0.04 | 0.25 ± 0 |
| T77476 | GUANINE NUCLEOTIDE BINDING PROTEIN (G PROTEIN), ALPHA 13 | 1.39 ± 0.41 | 1.59 ± 0.08 | 1.24 ± 0.14 |
| R35167 | BAI1-ASSOCIATED PROTEIN 2 | 1.46 ± 0.15 | 0.97 ± 0.02 | 1.32 ± 0.12 |
| H06909 | PROTEIN PHOSPHATASE 1, REGULATORY (INHIBITOR) SUBUNIT 12B | 1.34 ± 0.03 | 1.16 ± 0.01 | 1.08 ± 0.03 |
| H14999 | RAC/CDC42 GUANINE NUCLEOTIDE EXCHANGE FACTOR (GEF) 6 | 0.22 ± 0.13 | 0.27 ± 0.14 | 0.28 ± 0 |
| N77112 | IQ MOTIF CONTAINING GTPASE ACTIVATING PROTEIN 1 | 1.6 ± 0.35 | 1.54 ± 0.13 | 1.48 ± 0.04 |
| BI752921 | PLATELET-DERIVED GROWTH FACTOR RECEPTOR, BETA POLYPEPTIDE | 1.43 ± 0.09 | 1.03 ± 0.02 | 1.09 ± 0.07 |
| N99223 | INTEGRIN, ALPHA 9 | 1.46 ± 0.12 | 1.08 ± | 1.29 ± 0.01 |
| BI258438 | COFILIN 1 (NON-MUSCLE) | 1.24 ± 0.09 | 1.08 ± 0.09 | 1.42 ± 0.04 |
| BQ017489 | ACTININ, ALPHA 1 | 1.57 ± 0.22 | 1.24 ± 0.1 | 1.5 ± 0.1 |
| H51445 | LIM DOMAIN KINASE 1 | 1.3 ± 0 | 1.02 ± 0.11 | 1.12 ± 0.01 |
| AA010526 | GLUCOCORTICOID RECEPTOR DNA BINDING FACTOR 1 | 1.44 ± 0.14 | 1.03 ± 0.04 | 1.31 ± 0 |
| AA043909 | PTK2 PROTEIN TYROSINE KINASE 2 | 0.52 ± 0.11 | 0.52 ± 0.12 | 0.34 ± 0 |
For description see legend of table 5
Table 11.
TGF-β regulated genes (23) in A549 involved in Wnt signalling
| Acc No | DAVID Gene Name | 1 hr | 4 hr | 12 hr |
| H78804 | SMAD, MOTHERS AGAINST DPP HOMOLOG 3 (DROSOPHILA) | 1.22 ± 0.09 | 1.36 ± 0.02 | 1.15 ± 0.06 |
| AA029506 | CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE (CAM KINASE) II DELTA | 1.29 ± 0.22 | 1.41 ± 0.07 | 1.44 ± 0.02 |
| AA027986 | PROTEIN PHOSPHATASE 2 (FORMERLY 2A), CATALYTIC SUBUNIT, ALPHA ISOFORM | 1.24 ± 0.15 | 1.65 ± 0.03 | 1.34 ± 0.11 |
| AA029516 | TRANSCRIPTION FACTOR 7-LIKE 2 (T-CELL SPECIFIC, HMG-BOX) | 1.25 ± 0.02 | 1.41 ± 0.02 | 1.14 ± 0.2 |
| AA044665 | F-BOX AND WD-40 DOMAIN PROTEIN 11 | 1.09 ± 0.02 | 1.31 ± 0.02 | 1.07 ± 0.1 |
| AA030029 | PROTEIN KINASE C, ALPHA | 1.33 ± 0.23 | 1.56 ± 0.08 | 1.36 ± 0.03 |
| BE738680 | RAS HOMOLOG GENE FAMILY, MEMBER A | 1.24 ± 0.11 | 1.4 ± 0.06 | 1.51 ± 0.03 |
| R55134 | TRANSCRIPTION FACTOR 7-LIKE 1 (T-CELL SPECIFIC, HMG-BOX) | 1.45 ± 0.03 | 1.2 ± 0.1 | 1.27 ± 0.05 |
| W49562 | PHOSPHOLIPASE C, BETA 4 | 1.26 ± 0.25 | 1.27 ± 0.04 | 1.77 ± 0.27 |
| AA127934 | PROTEIN KINASE, X-LINKED | 1.21 ± 0.07 | 1.38 ± 0.01 | 1.35 ± 0.07 |
| H89206 | PROTEIN KINASE, CAMP-DEPENDENT, CATALYTIC, BETA | 1.18 ± 0.08 | 1.42 ± 0.04 | 1.25 ± 0.03 |
| N72642 | CALCYCLIN BINDING PROTEIN | 1.38 ± 0.01 | 1.5 ± 0.12 | 1.08 ± 0.06 |
| BM924824 | RAS-RELATED C3 BOTULINUM TOXIN SUBSTRATE 1 (RHO FAMILY, SMALL GTP BINDING PROTEIN RAC1) | 1.39 ± 0 | 1.41 ± 0.06 | 1.28 ± 0.18 |
| W51811 | WINGLESS-TYPE MMTV INTEGRATION SITE FAMILY, MEMBER 5A | 1.48 ± 0.1 | 1.54 ± 0.06 | 1.51 ± 0.19 |
| BM311217 | WINGLESS-TYPE MMTV INTEGRATION SITE FAMILY, MEMBER 4 | 1.63 ± | 0.97 ± | 1.5 ± 0.02 |
| R25275 | GLYCOGEN SYNTHASE KINASE 3 BETA | 1.15 ± 0.16 | 1.36 ± 0.04 | 1.23 ± 0.06 |
| AA152025 | FRIZZLED HOMOLOG 4 (DROSOPHILA) | 1.47 ± 0.28 | 1.56 ± 0.14 | 1.27 ± 0.12 |
| BM999610 | V-JUN SARCOMA VIRUS 17 ONCOGENE HOMOLOG (AVIAN) | 1.57 ± 0.26 | 2.13 ± 0.01 | 1.66 ± 0.18 |
| T75436 | MITOGEN-ACTIVATED PROTEIN KINASE 10 | 1.16 ± 0.17 | 1.22 ± 0.05 | 1.37 ± 0.07 |
| AA028175 | CULLIN 1 | 1.51 ± 0.15 | 1.15 ± 0.04 | 0.96 ± 0.02 |
| H70045 | CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE (CAM KINASE) II GAMMA | 1.45 ± 0.08 | 1.39 ± 0.16 | 1.21 ± 0.12 |
| R72188 | LOW DENSITY LIPOPROTEIN RECEPTOR-RELATED PROTEIN 5 | 1.27 ± 0.13 | 0.87 ± | 1.38 ± 0.02 |
| R14058 | PROTEIN KINASE C, BETA 1 | 1.54 ± 0.06 | 1.08 ± 0.01 | 1.15 ± 0 |
For description see legend of table 5
Effect of inhibiting the MAPK pathways on the TGF-β mediated gene regulation
TGF-β modulates gene expression through phosphorylation of SMAD proteins. However, several studies show a role for MAP kinase pathway in TGF-β mediated gene regulation both in a SMAD dependent and independent manner [18-20]. Hence, inhibitors of MAP kinase pathway components, SB203580 (p38), PD98059 (ERK) and JNK inhibitor 1-L form were used to assess the role of p38, ERK and JNK pathways in the regulation of gene expression by TGF-β. In A549 cells, regulation of IGFBP7 by TGF-β is independent of the p38, ERK and the JNK pathways. Induction of Integrin αV, MMP2, TMEPA and TGM2 by TGF-β is partially dependent on the ERK pathway; and MMP2, TGFBIP, TGM2 and TSP-1 regulation by TGF-β is partially dependent on the p38 pathway. The induction of Integrin αV is partially affected by blocking the JNK pathway (Fig. 5). In HPL1D cells, Intgerin αV, MMP2 and TGFBIP regulation by TGF-β is affected by blocking the p38 MAP kinase pathway, none of the gene regulations by TGF-β seem to be affected by blocking the ERK pathway and the induction of TMEPA by TGF-β is dependent on the JNK pathway (Fig. 6).
Figure 5.
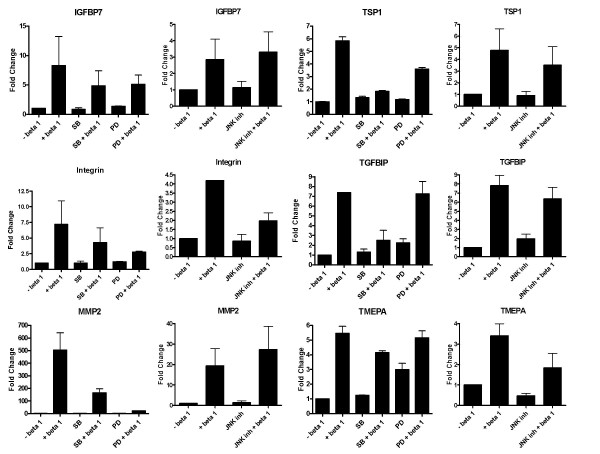
Effect of inhibition of the MAP kinase pathways on the regulation of gene expression by TGF-β in A549 cells. The cells were grown to 90% confluence, washed with serum free medium, treated with 10 μM p38 inhibitor (SB203580), ERK inhibitor (PD98059) or JNK inhibitor 1 hour prior to treatment with TGF-β. The cells were harvested after 18 hours following TGF-β treatment and processed for qRT-PCR as described in Figure 3.
Figure 6.
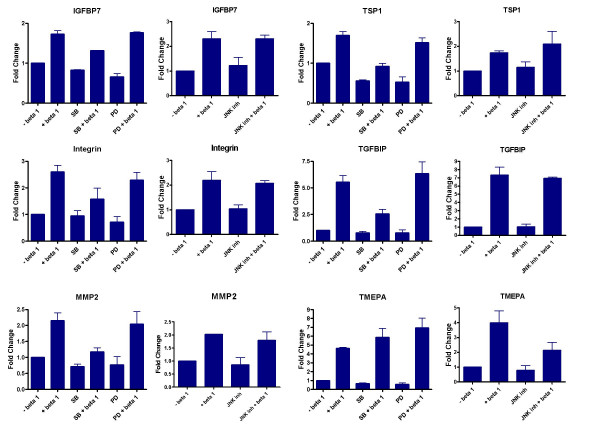
Effect of inhibition of the MAP kinase pathways on the regulation of gene expression by TGF-β in HPL1D cells. The cells were grown to 90% confluence, washed with serum free medium, treated with 10 μM p38 inhibitor (SB203580), ERK inhibitor (PD98059) or JNK inhibitor 1 hour prior to treatment with TGF-β. The cells were harvested after 18 hours following TGF-β treatment and processed for qRT-PCR as described in Figure 3.
Effect of blocking the Integrin-linked signalling pathway in A549 cells
Apart from the various signalling pathways and phenomena modulated by TGF-β in both normal and tumour cells, a pathway which is likely be differentially regulated is the Integrin-αV linked signalling pathway. Integrin αV is induced by TGF-β in A549 cells to about 3.5 fold as compared to 1.8 fold in HPL1D. Integrin αV is known to mediate some actions of TGF-β [21,22]. In order to test if some of the genes regulated by TGF-β are due to activation of Integrin αV, the activation of this pathway was blocked by treating the cells with 500 μg/ml of GRGDNP peptide, a known Integrin pathway inhibitor, prior to treatment with TGF-β. It was found that the induction of TSP-1, which is a TGF-β regulated gene only in A549 cells, is affected by blocking the integrin-linked signalling pathway (Figure 7 and Figure 8). Blocking this pathway had no effect on the regulation of the other genes tested. This suggests that the Integrin pathway is also responsible for differential regulation of some genes by TGF-β in the two cell-types, HPL1D and A549.
Figure 7.
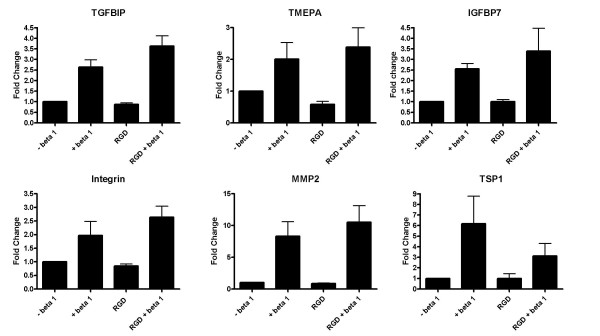
Effect of blocking the Integrin-linked signalling on the regulation of gene expression by TGF-β in A549 cells. The cells were grown to 90% confluence, washed with serum free medium, treated with 500 μg/ml GRGDNP peptide 3 hours prior to treatment with TGF-β. The cells were harvested after 12 hours following TGF-β treatment and processed for qRT-PCR as described in Figure 3.
Figure 8.
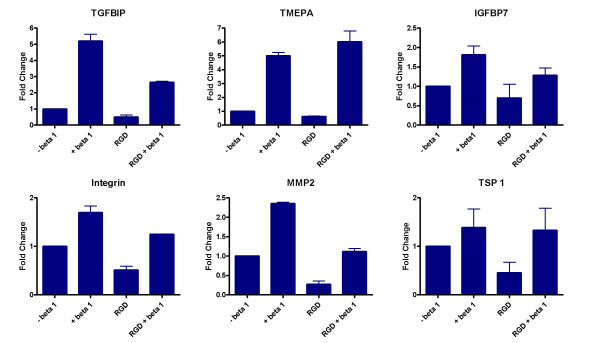
Effect of blocking the Integrin-linked signalling on the regulation of gene expression by TGF-β in HPL1D cells. The cells were grown to 90% confluence, washed with serum free medium, treated with 500 μg/ml GRGDNP peptide 3 hours prior to treatment with TGF-β. The cells were harvested after 12 hours following TGF-β treatment and processed for qRT-PCR as described in Figure 3.
Discussion
Most actions of TGF-β are brought about by regulation of gene expression. The genes, which are regulated and the way they are regulated are largely dependent on the cell-type under consideration. Over the past few years, there have been several independent transcriptome analyses of cells in response to TGF-β treatment. In cells of epithelial origin like human keratinocytes and mouse breast epithelial cells, TGF-β modulates genes involved in EMT [23,24]. In lung fibroblasts and A549 cells, TGF-β treatment results in the induction of extracellular matrix genes which are responsible for fibrosis as in the case of idiopathic pulmonary and alveolar epithelial fibrosis [25,26]. Studies using various cells such as cervical cancer cells, corneal epithelial cells, rat intestinal epithelial cells, and dermal fibroblasts show that regulation of gene expression by TGF-β is cell-type specific [27-30]. Considering the dual role of TGF-β on normal and transformed cells, wherein it confers growth inhibition and apoptosis to normal epithelial cells but aids growth and metastasis of tumour cells [4-7], it is essential to identify the genes and/or biochemical pathways regulated by TGF-β in normal and transformed cells. An understanding of these differences may allow identification of therapeutic targets for diseases involving TGF-β signalling pathway.
With this aim, expression profiling of genes in response to TGF-β was performed in a lung adenocarcinoma cell-line (A549) and a matched immortalized lung epithelial cell-line (HPL1D). The experimental design includes treatment of the respective cells with 5 ng/ml TGF-β in serum free conditions for 1, 4 and 12 hrs and extract RNA for microarray experiments. In our experiments, we have used two arrays for each RNA (time point) and hence essentially these are technical duplicates. As a consequence although duplicate arrays were performed for each time point, effectively the sample size equals to 1 at each time point since they are technical duplicates. Due to this, it is possible that many changes that are seen could be due to variations in the experimental protocols, rather than real biological differences. To overcome this, we have performed qRT-PCR validation of several genes on biological replicates that agrees with the microarray data for the respective genes. Our data showed similar regulation of 267 genes in HPL1D and A549 cells by TGF-β. This suggests that the genes commonly regulated in both HPL1D and A549 are not tumour specific. Some of these genes were also reported to be regulated by TGF-β in other studies using microarray in various cell-types [23,24,27] suggesting that these TGF-β regulated genes are not tumour cell specific but generally regulated by TGF-β. While some 1757 genes are exclusively regulated by TGF-β in A549, only 733 genes are exclusively regulated in HPL1D cells. The reasons for this differential response are not known. However, some of the genes exclusively regulated in A549 such as Integrin αV, thrombospondin 1, α2macroglobulin have been shown to aid tumour survival, maintenance and metastasis [31-33]. In contrast, in HPL1D, TGF-β regulates tumour suppressor genes like WT1, ECM proteins like collagen which are responsible for arrest of cell growth and apoptosis. This differential gene regulation in normal and tumour cells may explain the dual role of TGF-β in carcinogenesis. When the genes regulated by TGF-β in these two cell-lines were categorized based on their annotated functions, it was found that signalling pathways like MAP kinase, focal adhesion, Wnt signalling are regulated by TGF-β in both the cell-lines. On the other hand, Integrin αV was found to be differentially regulated in A549 and HPL1D cells.
TGF-β actions on cells are to a large extent are carried out by the phosphorylation of SMAD 2/3 by activated TGF-β type I receptor upon TGF-β signalling. Several genes that are transcriptionally regulated by TGF-β contain a SMAD complex binding element (SBE). However, many genes are reported to be regulated by TGF-β through an AP1 element [34,35]. Using SMAD2/SMAD3 knock out fibroblast cell-lines, SMAD 2 and 3 independent actions of TGF-β have been reported earlier [36]. In addition, SMAD independent signalling by TGF-β mediated by other pathways like the MAP kinases namely the p38, ERK and the JNK pathways is also reported [18-20,37-40]. Activation of the p38 and the JNK pathways by TGF-β involves a TGF-β activated kinase called TAK1 (reviewed in [41,42]). However, the ERK pathway activation by TGF-β is not well characterized. Also, cross talk between SMAD mediated signalling and PKC pathway has been reported [43]. Another pathway that has been implicated in TGF-β actions is the integrin signalling pathway. It has been shown that activation of integrin β 1 along with αV is important for the EMT mediated by TGF-β [22]. Although, Integrin αV is known to mediate some actions of TGF-β in some cell-types [21,22,44-46], the genes regulated by TGF-β through this pathway have not been characterized. Our data suggest substantial induction of integrin αV by TGF-β in only A549 cells and marginally in HPL1D cells. This could be an important difference between these two cell-lines for the regulation of gene expression mediated by TGF-β via this pathway. In order to understand the mechanism of regulation of some of the previously validated genes identified in the microarray experiments, specific inhibitors for the three branches of the MAP kinase pathway namely the p38, the ERK and the JNK pathways were used prior to TGF-β treatment of the cells and expression of these genes was studied by real time qRT-PCR estimation. The results suggested that in A549 cells, active MAP kinase pathways are involved in the regulation of more number of genes with relatively higher magnitude by TGF-β as compared to HPL1D cells (Fig. 5&6). There could be several reasons for this observation: 1] It is known that the MAP kinases are constitutively active in highly malignant cells due to autocrine activation of MAP kinase pathways [47], PI3K/Akt pathways [48], constitutive activation or amplification of receptors such as EGFR, PDGFR etc., mechanisms [49]. MAP kinase pathway involvement in the TGF-β mediated gene regulation is well established and the constitutive activation of MAP kinase pathway in transformed cells could be one of the reasons for more number of genes regulated by TGF-β in A549 cells. 2] It could be due to activation of Integrin pathway in transformed cells. It has been reported that Integrin mediated FAK/ILK activation is required for the regulation of EMT [22,50]. Our data suggesting regulation of Thrombospondin 1 which is an EMT marker [51] by TGF-β via integrin pathway, could be an example of this pathway's importance in TGF-β mediated gene regulation at least with respect to genes that mediate EMT. Taken together, our data highlights the importance of MAP kinase and integrin pathways in the regulation of majority of genes by TGF-β at least in transformed cells. The role of SMAD pathway in the regulation of these genes that are affected by the MAP kinase and integrin αV inhibitors is not known. It is possible that some of these regulations that depend on non-SMAD pathways may also need activated SMAD pathway. One difference between normal and tumour cells could be the absence of an intact SMAD signalling pathway in tumour cells. However, in A549 cells, no defects in the SMAD pathway have been reported. In addition, we confirmed the presence of intact SMAD pathway in A549 cells by SMAD dependent regulation of promoter activity by TGF-β (See additional file 5, Fig. S2). Based on our data, we propose that activated MAP kinase pathway could be one of the essential determining factors for the various differential actions of TGF-β in tumour cells.
A major question that remains to be addressed is whether the TGF-β mediated differential gene expression patterns in HPL1D and A549 cells is due to the distinct phenotypic characteristics of the respective cell-lines. From the previous studies, it is well established that TGF-β influences the growth and apoptosis of untransformed epithelial cells such as lung epithelial cells [52]. At least the growth inhibition by TGF-β has been less sensitive in the presence of serum [53] suggesting that activation of signalling pathways by serum may antagonize TGF-β mediated growth inhibition. Most apoptosis studies using TGF-β have also been carried out in minimal serum conditions (0.2–0.5 %). It remains to be established if apoptotic cell death mediated by TGF-β could be reversed in the presence of serum although it can be speculated that reversal of growth inhibition may allow survival of cells. One of the most important actions of the serum is activation of mitogenic pathways mediated by growth factors. Also, it has been shown that serum stimulation leads to activation of MAP kinase pathways [54]. Our data demonstrates that the effect of TGF-β is more pronounced in A549 cells as compared to HPL1D cells and one of the reasons for this could be due to constitutively active MAP kinase pathway in tumour cells. This is evident from our data that shows loss of TGF-β regulation of many genes following MAP kinase pathway specific inhibitors in A549 cells. A549 cells harbour a mutation at the 12th codon of K-ras proto-oncogene [55]. This mutation renders constitutive activation of RAS protein resulting in active MAP kinase pathway in these cells. In HPL1D cells, there is no report of any mutation in the RAS genes or constitutively active MAP kinase pathway. It is possible that this may be one of the major differences responsible for the differential regulation of genes by TGF-β in these cell-lines. HPL1D cells are growth inhibited by TGF-β [15]. The differential regulation of certain tumour suppressor genes (WT1, S100A2, TSSC1), cell cycle related genes (CDC34, CHEK1) and extracellular matrix genes (fibronectin, collagen IV and VIII) by TGF-β in HPL1D cells (see additional file 3, Table S3) points to a growth regulatory activity of TGF-β in these cells. On the other hand, as discussed previously, genes that are known to play pro-tumourigenic roles such as thrombospondin, integrins, transglutaminase, α2-macroglobulin etc. are regulated in A549 cells (see additional file 2, Table S2). Taken together, the differential regulation of genes in A549 and HPL1D cells could be due to pro-tumourigenic or growth inhibitory roles of TGF-β on these cells.
Conclusion
In conclusion, by microarray and real time RT-PCR experiments, we show that 1] TGF-β regulates more number of genes in transformed cells as compared to non-transformed cells; 2] evidence for differential regulation of gene expression in normal and tumour cells by TGF-β; and 3] that involvement of MAP kinase pathways may be one of the major mechanisms of TGF-β actions.
Methods
Cell-lines and cultures
A549, a lung adenocarcinoma cell-line was cultured in DMEM (Sigma-Aldrich, USA) with 10% foetal bovine serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin, 2.5 μg/ml fungizone (Invitrogen Life Sciences, USA). HPL1D, an immortalized lung epithelial cell-line was cultured in Ham's F-12 supplemented with 5% FBS, 5 μg/ml bovine insulin, 5 μg/ml human transferrin, 10-7M hydrocortisone, 2 × 10-10M tri-iodo thyronine and 20 ng/ml EGF [15]. All the cell-lines were maintained at 37°C in a humid atmosphere with 5% CO2.
Treatments
The cell-lines were grown to 90% confluence in the respective growth media followed by serum free washes (3 times) for 24 hours and then treated with 5 ng/ml TGF-β1 (R&D systems, USA) for different intervals of time. For signal transduction pathway inhibitor experiments, cells were pre-treated with 10 μM SB203580 (p38 inhibitor), 10 μM PD98050 (ERK inhibitor), and 10 μM JNK inhibitor I (L-form) (all from Calbiochem) for 1 hour, and 500 μg/ml GRGDNP peptide (custom synthesized) for 2–3 hours prior to TGF-β treatments.
RNA isolation, cDNA labelling and microarray analysis
Total RNA was isolated from cells using TRI reagent (Sigma-Aldrich, USA) according to manufacturer's protocol. The RNA was re-precipitated with 0.3 M sodium acetate (pH 5.2) and ethanol, further purified using RNAeasy columns (Qiagen, GmbH, Germany). The RNA quantity and quality were assessed by OD260 and OD280 measurements on a spectrophotometer and the integrity was determined by MOPS-formaldehyde gel. The labelling of the cDNA for microarray was done using Micromax Direct Labelling kit (Perkin Elmer Life Sciences Inc. USA) according to manufacturer's instructions. For each labelling reaction, 20 μg of total RNA was used. The control RNA was labelled with Cy3-dUTP and the TGF-β 1 treated RNA with Cy5-dUTP. Both the labelled products were mixed and precipitated with 0.3 M sodium acetate (pH 5.2) and ethanol. A small quantity of the precipitated labelled cDNA was assessed for labelling efficiency on a 1% agarose gel and scanned using the Typhoon 9210 scanner (GE Life Sciences). 160 μl of hybridisation buffer (Ultrahyb, Sigma-Aldrich, USA) was added to the probe, incubated at 75°C for 5 minutes and then added to the human 19 K array (University Health network, Toronto). The hybridisation was carried out in the GeneTAC Hyb Station (Genomic Solutions, UK) at 65° for 4 hours, 60° for 4 hours and 55° for 10 hours. The slides were then washed with medium stringency (2× SSC and 0.1% SDS), high stringency (0.1× SSC and 0.1% SDS) and post wash (0.1× SSC) buffers for 5 minutes each, dried and scanned using a scanner (Scanarray Express, Perkin Elmer Life Sciences, USA).
Microarray image and data analyses
All the image analyses have been done using the Quantarray software (Perkin Elmer Life Sciences, USA). Filtering and compilation of data have been done using Microsoft Excel and Microsoft Access. Spots of compromised quality and with low intensity were eliminated from the analysis. The data was normalized by LOWESS method (Avadis 3.1, Strand Life Sciences, India), Cy5:Cy3 ratios were established and log2 values were calculated. Hybridizations of RNA from each time point were performed on duplicate (technical duplicate) arrays and only those genes, which showed consistent regulation, have been considered for analysis. Genes which show log2ratios greater than 0.37 (1.3 fold induced) or less than -1.5 (3 fold down regulated) in the duplicate arrays at any one of the time points have been considered as regulated by TGF-β. The cut-off values for regulated genes were decided based on our observation of the data where genes that were known to be up regulated by TGF-β were showing > 1.3 and < 0.33 fold difference in the microarray.
Real Time PCR analyses
For cDNA synthesis, two micrograms of total RNA was reverse transcribed using the ABI cDNA Archive kit (Applied Biosystems, USA). Complementary DNA (cDNA) equivalent to 10 ng of total RNA was used for all the PCR reactions. The sequences of the primers are shown in Table 1. All the PCR reactions have been done using Dynamo SYBR green mix (Finnzymes, Finland) in ABI Prism 7900HT sequence detection system (Applied Biosystems, USA). The analysis has been done using SDS 2.1 software (Applied Biosystems, USA). For normalization of RT-PCR data, RPL35a expression (which was previously found to be unchanged in microarray experiments) was used and fold over control at each of the time point has been calculated as follows
| δCT = CTgene-CTRPL |
| δδCT = δCTtreated-δCTuntreated |
| Fold Change = 2-δδCT. |
Authors' contributions
PR designed and executed the experiments, interpreted the data and prepared the manuscript; AA, RB and RKRK analysed the microarray data and prepared the gene lists and figures; AC performed the real time PCR experiments and analysis of data; TT provided the support in the design of experiments and PK conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
List of genes commonly regulated by TGF-β in HPL1D and A549 cell-lines. A list of genes, which are regulated by TGF-β in both HPL1D and A549 cell-lines along with the fold changes at 1, 4 and 12 hrs of treatment.
List of genes regulated by TGF-β in only A549 cell-line. A list of genes, which are regulated by TGF-β in A549 alone along with the fold changes at 1, 4 and 12 hrs of treatment.
List of genes regulated by TGF-β only in HPL1D cell-line. A list of genes, which are regulated by TGF-β in HPL1D cell-line alone along with the fold changes at 1, 4 and 12 hrs of treatment.
Semi-quantitative RT-PCR analyses of selected genes with respect to regulation by TGF-β in A549 and HPL1D cells. The respective cell-lines were grown to 90% confluence (as described in methods section), washed with serum free medium, treated with 5 ng/ml TGF-β 1 for 1, 4, 6, 12 and 24 hours. Each treatment also has untreated cells as control at each time-point. Two microgram of total RNA from each treatment was reverse transcribed and cDNA equivalent to 20 ng total RNA was used for the PCR reactions. All PCR reactions were done under non saturating conditions. The products were resolved on 2% agarose gel and the gel pictures were taken on Kodak Image station 440CF. The band intensities were quantified using Kodak 1D 3.6 software. A, ethidium bromide staining pattern of the PCR products. B and C graphs representing the fold change over untreated controls after normalization with the expression of RPL35a, in HPL1D and A549 cells respectively.
Induction of pSBE-Luc activity by TGF-β in A549 cells. For SBE-luc induction by TGF-β in A549 cells, twenty five thousand cells were plated in 24 well dishes 16–24 hours prior to transfection. 200 ng of pSBE-luc plasmid and 1.25 ng of pRL-CMV construct (Renilla luciferase, for transfection normalization) were transfected using Effectene reagent (Qiagen GmbH, Germany) in serum free conditions for 12 hrs. The cells were recovered in medium containing 10% FBS for 24 hours, washed with serum free medium for 24 hours (3 changes) and then treated with 5 ng/ml TGF-β for 18 hours. The cells were then lysed and lysates were used for dual-luciferase assay (Promega inc, USA). The ratio of the firefly-luciferase to renilla-luciferase has been plotted on the y-axis.
Acknowledgments
Acknowledgements
We thank Dr. Karunagaran and Dr Bert Vogelstein for pSBE4-BV/Luc construct. We acknowledge the help of Dr. Gayathri Ramakrishnan and Praveen Arany for useful discussions, T.N. Vivek for the help with inhibitor experiments and P. Sreekanth Reddy for the help with Microarray and real time PCR experiments. We thank Dr. Ramesh Hariharan, Strand Life Sciences, Bangalore for Avadis microarray analysis software and M/s Unichem Laboratories, IISc for the GRGDNP peptide. Indian Council of Medical Research, India, funded this study. The infrastructural support received from University Grants Commission and Department of Science and Technology (DST FIST), Govt. of India, New Delhi is acknowledged.
Contributor Information
Prathibha Ranganathan, Email: pratiba@mrdg.iisc.ernet.in.
Animesh Agrawal, Email: animesh.agrawal@gmail.com.
Raghu Bhushan, Email: raghubhushanin@yahoo.co.in.
Aravinda K Chavalmane, Email: aravi_ck@yahoo.com.
Ravi Kiran Reddy Kalathur, Email: ravi@titus.u-strasbg.fr.
Takashi Takahashi, Email: tak@med.nagoya-u.ac.jp.
Paturu Kondaiah, Email: paturu@mrdg.iisc.ernet.in.
References
- Kulkarni AB, Thyagarajan T, Letterio JJ. Function of cytokines within the TGF-beta superfamily as determined from transgenic and gene knockout studies in mice. Curr Mol Med. 2002;2:303–327. doi: 10.2174/1566524024605699. [DOI] [PubMed] [Google Scholar]
- Levy L, Hill CS. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006;17:41–58. doi: 10.1016/j.cytogfr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:8621–8623. doi: 10.1073/pnas.1633291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle SJ, Hoying JB, Boivin GP, Ormsby I, Gartside PS, Doetschman T. Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59:3379–3386. [PubMed] [Google Scholar]
- Siegel PM, Shu W, Cardiff RD, Muller WJ, Massague J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc Natl Acad Sci U S A. 2003;100:8430–8435. doi: 10.1073/pnas.0932636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Muraoka-Cook RS, Dumont N, Arteaga CL. Dual role of transforming growth factor beta in mammary tumorigenesis and metastatic progression. Clin Cancer Res. 2005;11:937s–43s. [PubMed] [Google Scholar]
- Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002;109:1607–1615. doi: 10.1172/JCI200215333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka RS, Dumont N, Ritter CA, Dugger TC, Brantley DM, Chen J, Easterly E, Roebuck LR, Ryan S, Gotwals PJ, Koteliansky V, Arteaga CL. Blockade of TGF-{beta} inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest. 2002;109:1551–1559. doi: 10.1172/JCI200215234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao M, Williams K, Bhowmick NA, Hayward SW. Transforming Growth Factor-{beta} Promotes Invasion in Tumorigenic but not in Nontumorigenic Human Prostatic Epithelial Cells. Cancer Res. 2006;66:8007–8016. doi: 10.1158/0008-5472.CAN-05-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LR, Romer J, Ronne E, Ellis V, Blasi F, Dano K. Urokinase-receptor biosynthesis, mRNA level and gene transcription are increased by transforming growth factor beta 1 in human A549 lung carcinoma cells. Embo J. 1991;10:3399–3407. doi: 10.1002/j.1460-2075.1991.tb04904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J, Blasi F, Leof EB, Moses HL. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988;106:451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J, Raghow R, Sawdey M, Loskutoff DJ, Postlethwaite AE, Kang AH, Moses HL. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988;263:3111–3115. [PubMed] [Google Scholar]
- Samuel SK, Hurta RA, Kondaiah P, Khalil N, Turley EA, Wright JA, Greenberg AH. Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-beta 1 (Ser223, 225) Embo J. 1992;11:1599–1605. doi: 10.1002/j.1460-2075.1992.tb05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A, Kondo M, Saito T, Yatabe Y, Kobayashi T, Okamoto M, Suyama M, Takahashi T, Takahashi T. Establishment of human peripheral lung epithelial cell lines (HPL1) retaining differentiated characteristics and responsiveness to epidermal growth factor, hepatocyte growth factor, and transforming growth factor beta1. Cancer Res. 1997;57:4898–4904. [PubMed] [Google Scholar]
- Kim SJ, Jeang KT, Glick AB, Sporn MB, Roberts AB. Promoter sequences of the human transforming growth factor-beta 1 gene responsive to transforming growth factor-beta 1 autoinduction. J Biol Chem. 1989;264:7041–7045. [PubMed] [Google Scholar]
- Dennis G, Sherman B, Hosack D, Yang J, Gao W, Lane HC, Lempicki R. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biology. 2003;4:P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. Embo J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonson T, Heidenblad M, Hakansson P, Gorunova L, Johansson B, Fioretos T, Hoglund M. Pancreatic carcinoma cell lines with SMAD4 inactivation show distinct expression responses to TGFB1. Genes Chromosomes Cancer. 2003;36:340–352. doi: 10.1002/gcc.10179. [DOI] [PubMed] [Google Scholar]
- Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. alpha(v)beta(3) Integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J Biol Chem. 2004;279:37726–37733. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin beta 1 signaling is necessary for transforming growth factor-beta activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- Xie L, Law BK, Aakre ME, Edgerton M, Shyr Y, Bhowmick NA, Moses HL. Transforming growth factor beta-regulated gene expression in a mouse mammary gland epithelial cell line. Breast Cancer Res. 2003;5:R187–98. doi: 10.1186/bcr640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proc Natl Acad Sci U S A. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzoni E, Abraham D, Howat S, Shi-Wen X, Sestini P, Bou-Gharios G, Wells A, Veeraraghavan S, Nicholson A, Denton C, Leask A, Pearson J, Black C, Welsh K, du Bois R. Gene expression profiling reveals novel TGFbeta targets in adult lung fibroblasts. Respiratory Research. 2004;5:24. doi: 10.1186/1465-9921-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating D, Sadlier D, Patricelli A, Smith S, Walls D, Egan J, Doran P. Microarray identifies ADAM family members as key responders to TGF-beta1 in alveolar epithelial cells. Respiratory Research. 2006;7:114. doi: 10.1186/1465-9921-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloth JN, Fleuren GJ, Oosting J, de Menezes RX, Eilers PH, Kenter GG, Gorter A. Substantial changes in gene expression of Wnt, MAPK and TNFalpha pathways induced by TGF-beta1 in cervical cancer cell lines. Carcinogenesis. 2005;26:1493–1502. doi: 10.1093/carcin/bgi110. [DOI] [PubMed] [Google Scholar]
- Hayashida-Hibino S, Watanabe H, Nishida K, Tsujikawa M, Tanaka T, Hori Y, Saishin Y, Tano Y. The Effect of TGF-{beta}1 on Differential Gene Expression Profiles in Human Corneal Epithelium Studied by cDNA Expression Array. Invest Ophthalmol Vis Sci. 2001;42:1691–1697. [PubMed] [Google Scholar]
- Cao Y, Chen L, Zhang W, Liu Y, Papaconstantinou HT, Bush CR, Townsend JCM, Thompson EA, Ko TC. IDENTIFICATION OF APOPTOTIC GENES MEDIATING TRANSFORMING GROWTH FACTOR (TGF)-{beta}/Smad3-INDUCED CELL DEATH IN INTESTINAL EPITHELIAL CELLS USING A GENOMIC APPROACH. Am J Physiol Gastrointest Liver Physiol. 2006. p. 437.2005. [DOI] [PubMed]
- Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- Wang TN, Qian X, Granick MS, Solomon MP, Rothman VL, Berger DH, Tuszynski GP. Thrombospondin-1 (TSP-1) Promotes the Invasive Properties of Human Breast Cancer. Journal of Surgical Research. 1996;63:39. doi: 10.1006/jsre.1996.0219. [DOI] [PubMed] [Google Scholar]
- Harthun NL, Weaver AM, Brinckerhoff LH, Deacon DH, Gonias SL, Slingluff CL., Jr. Activated alpha 2-macroglobulin reverses the immunosuppressive activity in human breast cancer cell-conditioned medium by selectively neutralizing transforming growth factor-beta in the presence of interleukin-2. J Immunother. 1998;21:85–94. doi: 10.1097/00002371-199803000-00001. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Requirement of Ras/MAPK Pathway Activation by Transforming Growth Factor beta for Transforming Growth Factor beta 1 Production in a Smad-dependent Pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-[beta]-induced transcription. Nature. 1998;394:909. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]
- Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lan HY, Glushakova O, Zhu HJ, Kang DH, Schreiner GF, Bottinger EP, Johnson RJ, Sautin YY. Role of ERK1/2 and p38 mitogen-activated protein kinases in the regulation of thrombospondin-1 by TGF-beta1 in rat proximal tubular cells and mouse fibroblasts. J Am Soc Nephrol. 2005;16:899–904. doi: 10.1681/ASN.2004080689. [DOI] [PubMed] [Google Scholar]
- Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- Maliekal TT, Anto RJ, Karunagaran D. Differential activation of Smads in HeLa and SiHa cells that differ in their response to transforming growth factor-beta. J Biol Chem. 2004;279:36287–36292. doi: 10.1074/jbc.M404568200. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Yakymovych I, Ten Dijke P, Heldin CH, Souchelnytskyi S. Regulation of Smad signaling by protein kinase C. Faseb J. 2001;15:553–555. doi: 10.1096/fj.00-0474fje. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin in the establishment of autocrine TGF-beta signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126:1761–1769. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- Fjellbirkeland L, Cambier S, Broaddus VC, Hill A, Brunetta P, Dolganov G, Jablons D, Nishimura SL. Integrin alphavbeta8-mediated activation of transforming growth factor-beta inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163:533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Vara JAF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treatment Reviews. 2004;30:193. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Saxena A, Clark WC, Robertson JT, Oldfield EH, Aaronson SA, Ali IU. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52:4550–4553. [PubMed] [Google Scholar]
- Lee YI, Kwon YJ, Joo CK. Integrin-linked kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition. Biochem Biophys Res Commun. 2004;316:997–1001. doi: 10.1016/j.bbrc.2004.02.150. [DOI] [PubMed] [Google Scholar]
- Chagraoui J, Lepage-Noll A, Anjo A, Uzan G, Charbord P. Fetal liver stroma consists of cells in epithelial-to-mesenchymal transition. Blood. 2003;101:2973–2982. doi: 10.1182/blood-2002-05-1341. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K, Osada H, Masuda A, Kondo M, Saito T, Yatabe Y, Takagi K, Takahashi T, Takahashi T. Induction of apoptosis by Smad3 and down-regulation of Smad3 expression in response to TGF-beta in human normal lung epithelial cells. Oncogene. 1998;17:1743–1747. doi: 10.1038/sj.onc.1202052. [DOI] [PubMed] [Google Scholar]
- Danielpour D, Dart LL, Flanders KC, Roberts AB, Sporn MB. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989;138:79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Seger R, Diltz CD, Krebs EG, Fischer EH. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci U S A. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela DM, Groffen J. Four human carcinoma cell lines with novel mutations in position 12 of c-K-ras oncogene. Nucl Acids Res. 1986;14:843–851. doi: 10.1093/nar/14.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes commonly regulated by TGF-β in HPL1D and A549 cell-lines. A list of genes, which are regulated by TGF-β in both HPL1D and A549 cell-lines along with the fold changes at 1, 4 and 12 hrs of treatment.
List of genes regulated by TGF-β in only A549 cell-line. A list of genes, which are regulated by TGF-β in A549 alone along with the fold changes at 1, 4 and 12 hrs of treatment.
List of genes regulated by TGF-β only in HPL1D cell-line. A list of genes, which are regulated by TGF-β in HPL1D cell-line alone along with the fold changes at 1, 4 and 12 hrs of treatment.
Semi-quantitative RT-PCR analyses of selected genes with respect to regulation by TGF-β in A549 and HPL1D cells. The respective cell-lines were grown to 90% confluence (as described in methods section), washed with serum free medium, treated with 5 ng/ml TGF-β 1 for 1, 4, 6, 12 and 24 hours. Each treatment also has untreated cells as control at each time-point. Two microgram of total RNA from each treatment was reverse transcribed and cDNA equivalent to 20 ng total RNA was used for the PCR reactions. All PCR reactions were done under non saturating conditions. The products were resolved on 2% agarose gel and the gel pictures were taken on Kodak Image station 440CF. The band intensities were quantified using Kodak 1D 3.6 software. A, ethidium bromide staining pattern of the PCR products. B and C graphs representing the fold change over untreated controls after normalization with the expression of RPL35a, in HPL1D and A549 cells respectively.
Induction of pSBE-Luc activity by TGF-β in A549 cells. For SBE-luc induction by TGF-β in A549 cells, twenty five thousand cells were plated in 24 well dishes 16–24 hours prior to transfection. 200 ng of pSBE-luc plasmid and 1.25 ng of pRL-CMV construct (Renilla luciferase, for transfection normalization) were transfected using Effectene reagent (Qiagen GmbH, Germany) in serum free conditions for 12 hrs. The cells were recovered in medium containing 10% FBS for 24 hours, washed with serum free medium for 24 hours (3 changes) and then treated with 5 ng/ml TGF-β for 18 hours. The cells were then lysed and lysates were used for dual-luciferase assay (Promega inc, USA). The ratio of the firefly-luciferase to renilla-luciferase has been plotted on the y-axis.


