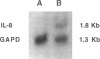
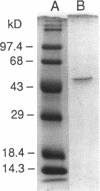
Abstract
Respiratory epithelial cells play a crucial role in the inflammatory response during Pseudomonas aeruginosa infection in the lungs of patients with cystic fibrosis. In this study, we determined whether the binding of specific Pseudomonas gene products (pilin, flagellin) to their receptors on respiratory epithelial cells would result in production of the neutrophil chemoattractant IL-8. Piliated wild-type organisms, purified pili, or antibody to the pilin receptor (asialoGM1) evoked significant production of IL-8 by immortalized airway epithelial cells, whereas nonpiliated organisms were less able to bind to respiratory epithelial cells and stimulated much less IL-8 secretion (P < 0.01). A piliated, nonflagellated strain was also associated with decreased binding and a diminished level of IL-8 production when compared to wild-type organisms. Isogenic, nonadherent rpoN mutants, lacking pilin and flagellin, did not bind or elicit an IL-8 response. In addition, the IL-8 response was four-fold higher in a cystic fibrosis cell line compared with its corrected cell line. The Pseudomonas autoinducer, an exoproduct secreted during chronic infection, was found to stimulate IL-8 in a dose-dependent manner. P. aeruginosa adhesins, which are necessary for initial infection, directly stimulate IL-8 production by respiratory epithelial cells and therefore play a major role in the pathogenesis of Pseudomonas infection in patients with cystic fibrosis. The inflammatory response is subsequently perpetuated by Pseudomonas autoinducer which is secreted during chronic infection.
Full text
PDF


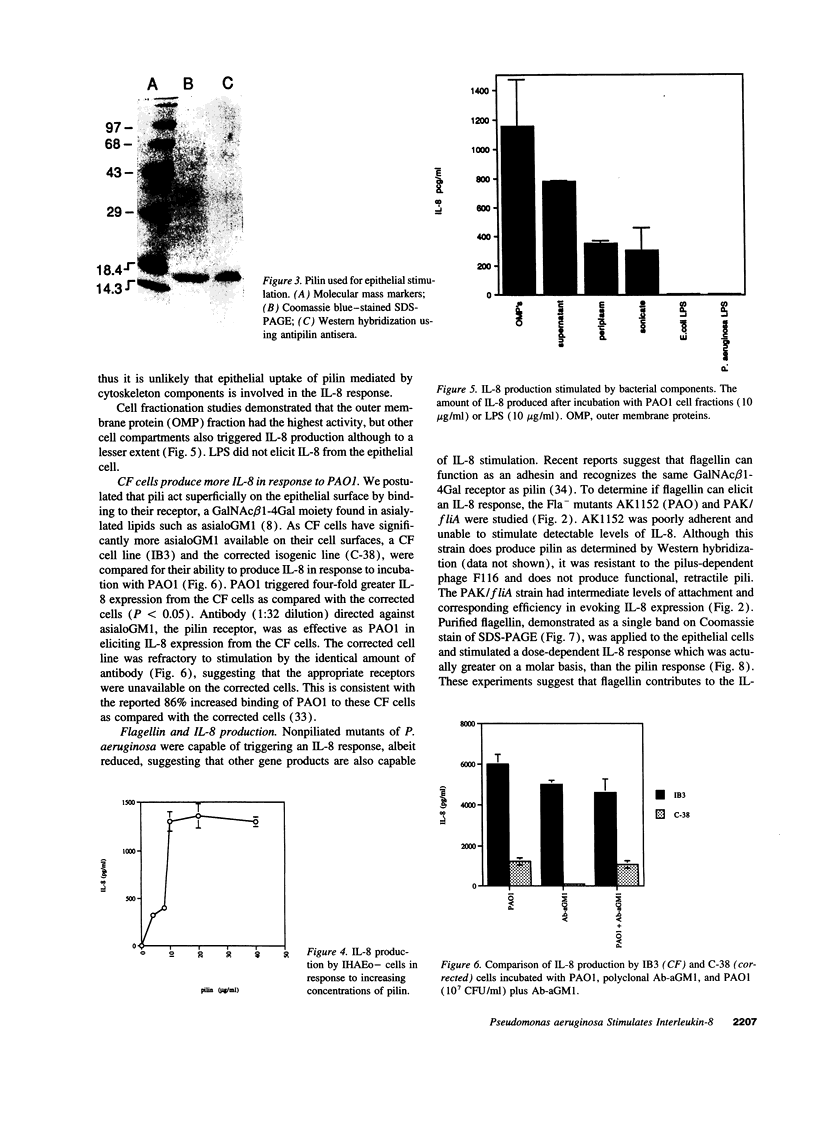



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agace W. W., Hedges S. R., Ceska M., Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J Clin Invest. 1993 Aug;92(2):780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J. S., Dawson M., Drake D., Montie T. C. Electrophoretic separation and molecular weight characterization of Pseudomonas aeruginosa H-antigen flagellins. Infect Immun. 1985 Sep;49(3):770–774. doi: 10.1128/iai.49.3.770-774.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore R. S., Christie C. D., Smith G. J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am Rev Respir Dis. 1989 Dec;140(6):1650–1661. doi: 10.1164/ajrccm/140.6.1650. [DOI] [PubMed] [Google Scholar]
- Barasch J., Kiss B., Prince A., Saiman L., Gruenert D., al-Awqati Q. Defective acidification of intracellular organelles in cystic fibrosis. Nature. 1991 Jul 4;352(6330):70–73. doi: 10.1038/352070a0. [DOI] [PubMed] [Google Scholar]
- Cacalano G., Kays M., Saiman L., Prince A. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmolar conditions and is regulated by genes involved in alginate expression. J Clin Invest. 1992 Jun;89(6):1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Preston A. M., Takeuchi E., Kenney J., Boxer L. A., Remick D. G. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993 Dec 5;268(34):25568–25576. [PubMed] [Google Scholar]
- Dean T. P., Dai Y., Shute J. K., Church M. K., Warner J. O. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res. 1993 Aug;34(2):159–161. doi: 10.1203/00006450-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Drake D., Montie T. C. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol. 1988 Jan;134(1):43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M. F., Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993 Nov;61(11):4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinha M. A., Conway B. D., Glasier L. M., Ellert N. W., Irvin R. T., Sherburne R., Paranchych W. Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect Immun. 1994 Oct;62(10):4118–4123. doi: 10.1128/iai.62.10.4118-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. R., Afione S. A., Conrad C., McGrath S. A., Solow R., Oka H., Zeitlin P. L., Guggino W. B., Carter B. J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10613–10617. doi: 10.1073/pnas.90.22.10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Nair G., Schweizer H. P. Construction and characterization of chromosomal insertional mutations of the Pseudomonas aeruginosa exoenzyme S trans-regulatory locus. Infect Immun. 1994 Feb;62(2):554–563. doi: 10.1128/iai.62.2.554-563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W. Composition and molecular weight of pili purified from Pseudomonas aeruginosa K. J Bacteriol. 1977 Jul;131(1):259–269. doi: 10.1128/jb.131.1.259-269.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert D. C., Basbaum C. B., Welsh M. J., Li M., Finkbeiner W. E., Nadel J. A. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5951–5955. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Imundo L., Barasch J., Prince A., Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Massion P. P., Inoue H., Richman-Eisenstat J., Grunberger D., Jorens P. G., Housset B., Pittet J. F., Wiener-Kronish J. P., Nadel J. A. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Invest. 1994 Jan;93(1):26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvaney N. G., Nakamura H., Birrer P., Hébert C. A., Wong W. L., Alphonso M., Baker J. B., Catalano M. A., Crystal R. G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992 Oct;90(4):1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., McElvaney N. G., Crystal R. G. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992 May;89(5):1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff R. M., Wretlind B., Vasil M. L. Mutations in the hemolytic-phospholipase C operon result in decreased virulence of Pseudomonas aeruginosa PAO1 grown under phosphate-limiting conditions. Infect Immun. 1989 May;57(5):1369–1373. doi: 10.1128/iai.57.5.1369-1373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton J. M. F116: a DNA bacteriophage specific for the pili of Pseudomonas aeruginosa strain PAO. Virology. 1973 Oct;55(2):558–560. doi: 10.1016/0042-6822(73)90203-1. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Koo L., Ishimoto K. S., Totten P. A., Lara J. C., Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991 Apr;59(4):1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiman L., Ishimoto K., Lory S., Prince A. The effect of piliation and exoproduct expression on the adherence of Pseudomonas aeruginosa to respiratory epithelial monolayers. J Infect Dis. 1990 Mar;161(3):541–548. doi: 10.1093/infdis/161.3.541. [DOI] [PubMed] [Google Scholar]
- Saiman L., Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Invest. 1993 Oct;92(4):1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. A., Ramphal R., Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992 Sep;60(9):3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnbach M. N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol Microbiol. 1992 Feb;6(4):459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Tang H., Kays M., Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995 Apr;63(4):1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcellos C. A., Allen P. G., Wohl M. E., Drazen J. M., Janmey P. A., Stossel T. P. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994 Feb 18;263(5149):969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993 Jul 2;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]
- Zar H., Saiman L., Quittell L., Prince A. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. J Pediatr. 1995 Feb;126(2):230–233. doi: 10.1016/s0022-3476(95)70549-x. [DOI] [PubMed] [Google Scholar]