Abstract
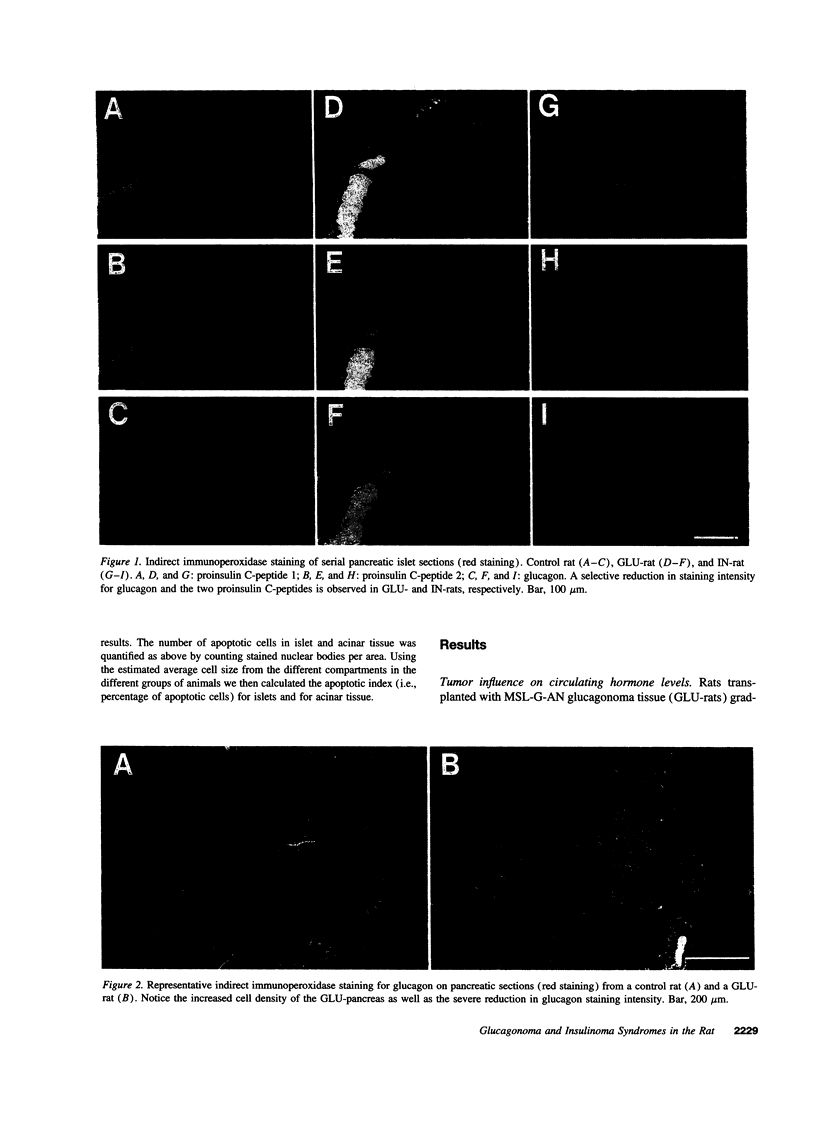
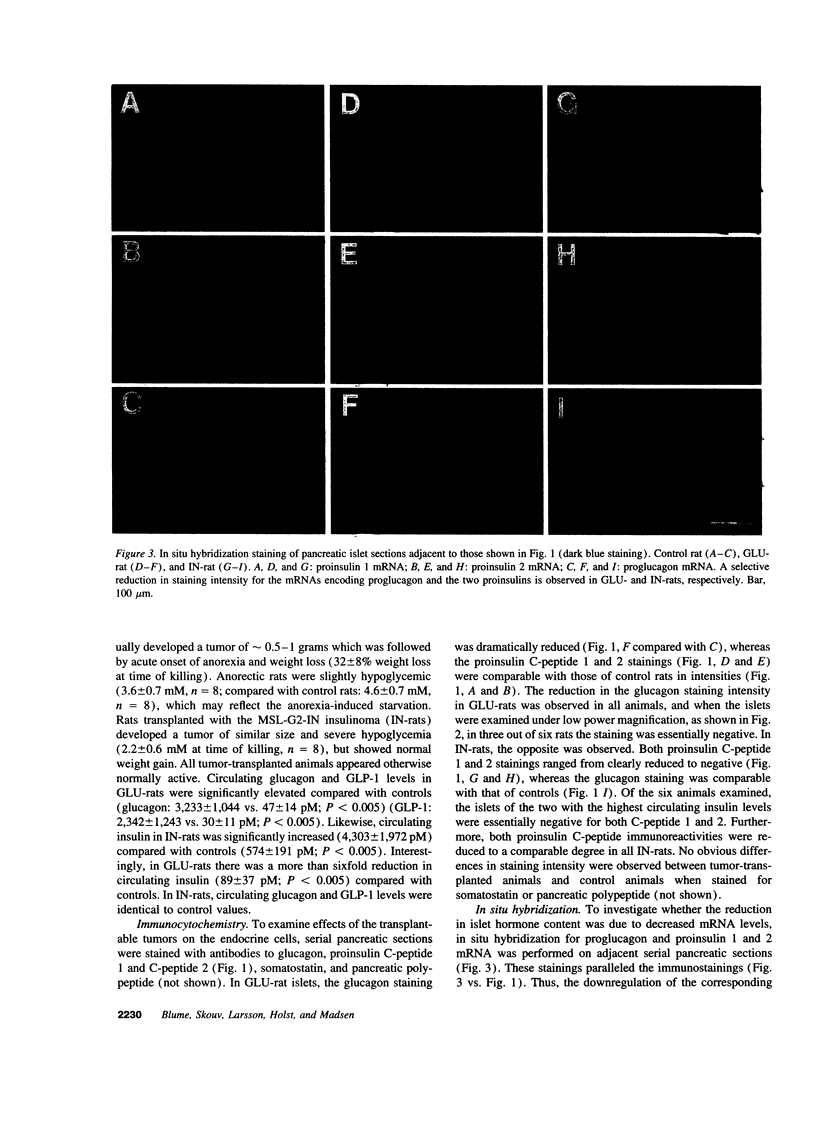
Effects of transplantable rat insulinomas (IN) and glucagonomas (GLU) on the endogenous pancreas were analyzed using morphometry, immunocytochemistry, in situ hybridization, and staining for apoptotic cells. Hyperinsulinemia (IN-rats) and hyper-GLP-1/glucagonemia (GLU-rats) were both associated with marked islet atrophy (67 and 76% of control average planimetrical islet area, respectively). Selective islet B cell inhibition of proinsulin (I and II) genes as well as of expression of the insulin gene transcription factor, IPF1/STF1, was found in IN-rats. Moreover, these islets were characterized by significant B cells apoptosis in the absence of infiltrating lymphocytes. In GLU-rats selective islet A cell inhibition was observed at the level of glucagon mRNA. These islets contained small, highly condensed but clearly active B cells with prominent IPF1/STF1-positive nuclei, surrounded by densely packed glucagon-negative cells with reduced cytoplasm. Furthermore, an active apoptotic process was found exclusively in the exocrine pancreas of GLU-rats. Thus, in IN-rats, islet B cell mass reduction is distinguished by non-immune-mediated programmed cell death, while GLU-rats exhibit A cell mass reduction by cytoplasmic retraction and selective exocrine apoptosis.
Full text
PDF

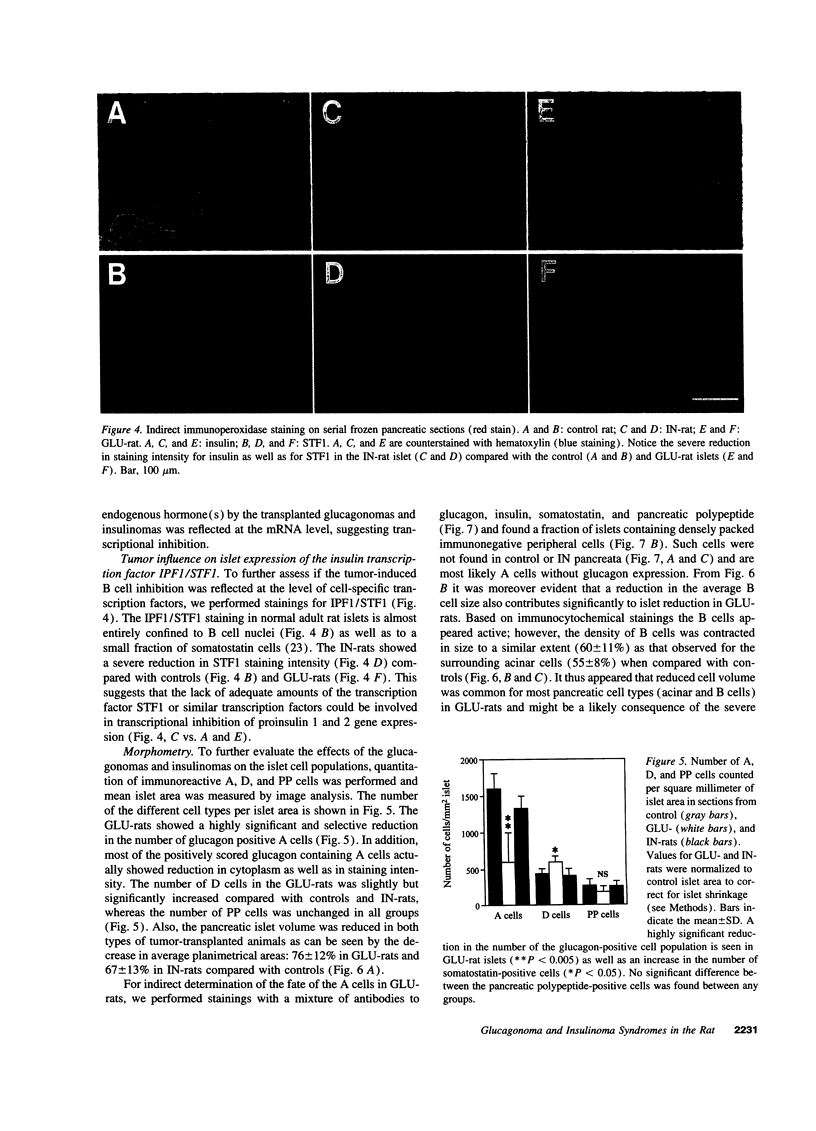
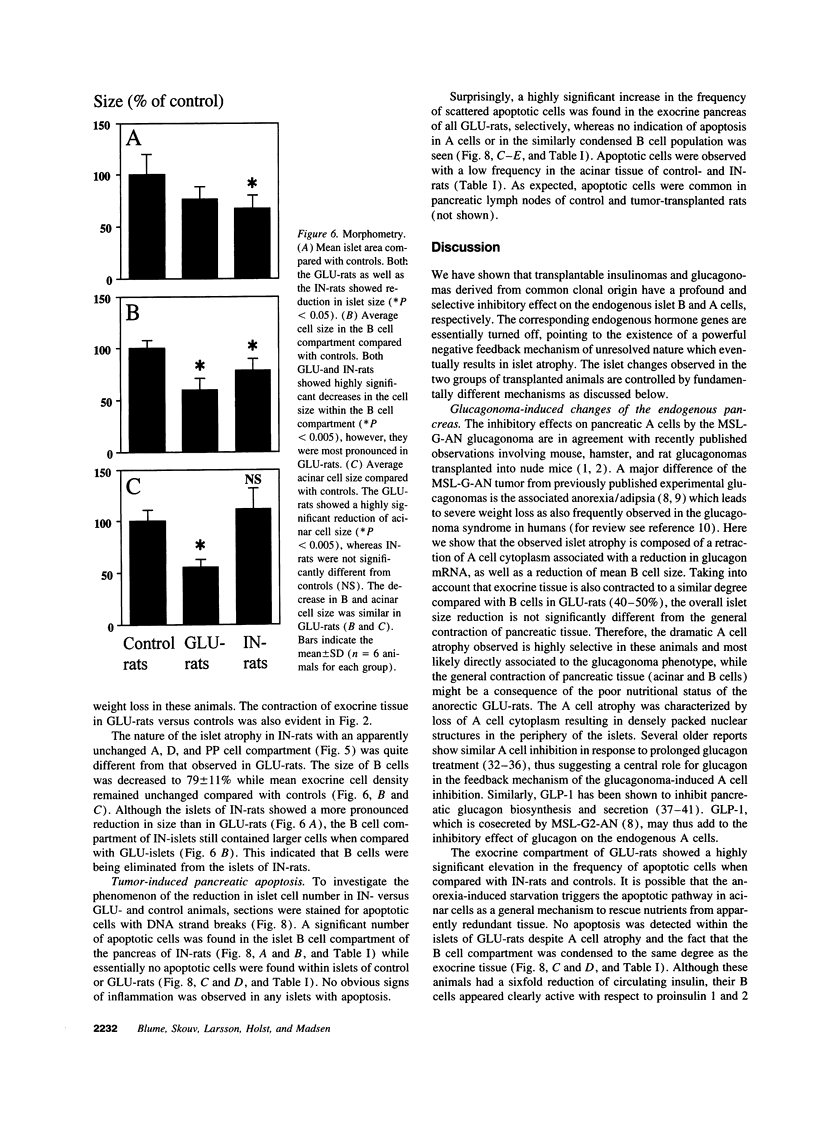
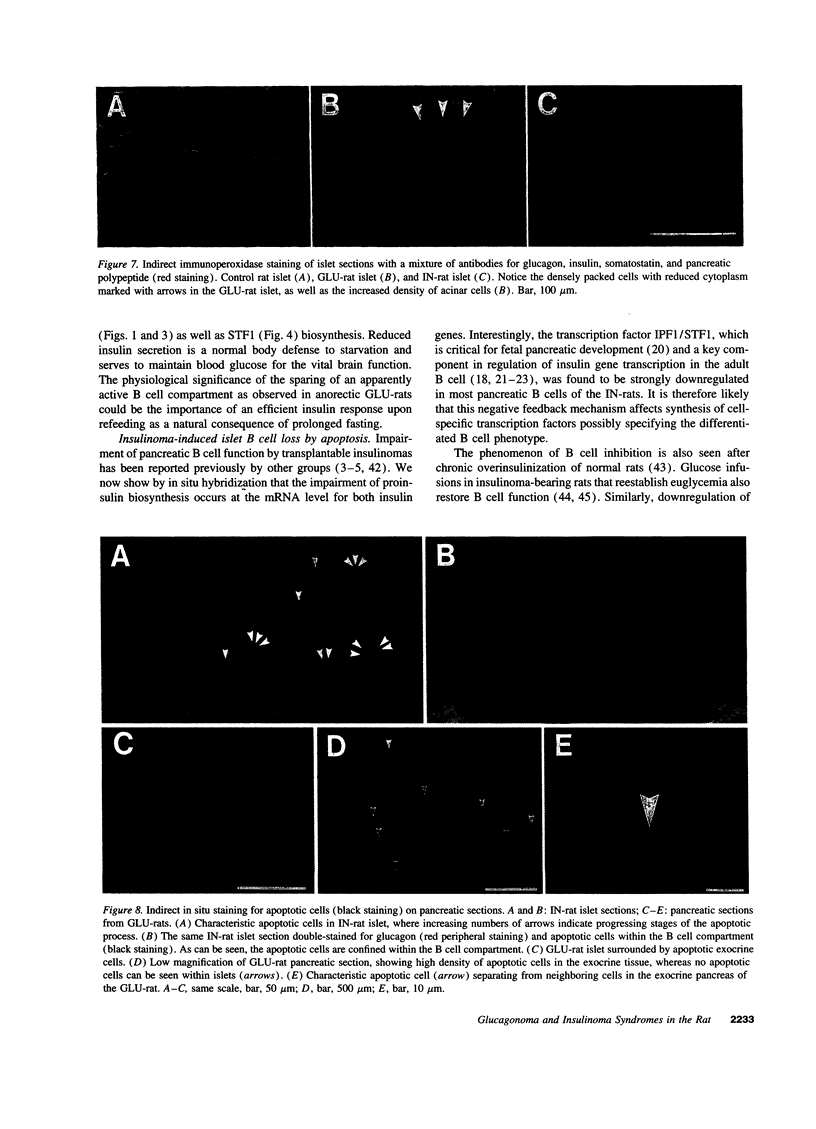


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam T., Chen L., Ogawa A., Leffert J. D., Unger R. H., Luskey K. L. Coordinate regulation of amylin and insulin expression in response to hypoglycemia and fasting. Diabetes. 1992 Apr;41(4):508–514. doi: 10.2337/diab.41.4.508. [DOI] [PubMed] [Google Scholar]
- Blume N., Petersen J. S., Andersen L. C., Kofod H., Dyrberg T., Michelsen B. K., Serup P., Madsen O. D. Immature transformed rat islet beta-cells differentially express C-peptides derived from the genes coding for insulin I and II as well as a transfected human insulin gene. Mol Endocrinol. 1992 Feb;6(2):299–307. doi: 10.1210/mend.6.2.1569972. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S. Regulation of pancreatic beta-cell mass in vivo. Recent Prog Horm Res. 1994;49:91–104. doi: 10.1016/b978-0-12-571149-4.50008-8. [DOI] [PubMed] [Google Scholar]
- Bursch W., Paffe S., Putz B., Barthel G., Schulte-Hermann R. Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis. 1990 May;11(5):847–853. doi: 10.1093/carcin/11.5.847. [DOI] [PubMed] [Google Scholar]
- Chen L., Komiya I., Inman L., O'Neil J., Appel M., Alam T., Unger R. H. Effects of hypoglycemia and prolonged fasting on insulin and glucagon gene expression. Studies with in situ hybridization. J Clin Invest. 1989 Aug;84(2):711–714. doi: 10.1172/JCI114219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Lee Y. C., Asa S. L., Brubaker P. L. Inhibition of pancreatic glucagon gene expression in mice bearing a subcutaneous glucagon-producing GLUTag transplantable tumor. Mol Endocrinol. 1992 Dec;6(12):2175–2184. doi: 10.1210/mend.6.12.1491697. [DOI] [PubMed] [Google Scholar]
- Ehrlich P., Tucker D., Asa S. L., Brubaker P. L., Drucker D. J. Inhibition of pancreatic proglucagon gene expression in mice bearing subcutaneous endocrine tumors. Am J Physiol. 1994 Nov;267(5 Pt 1):E662–E671. doi: 10.1152/ajpendo.1994.267.5.E662. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Scaglia L., Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995 Mar;44(3):249–256. doi: 10.2337/diab.44.3.249. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N., Le Sauter J., Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993 Jan;264(1 Pt 2):R116–R122. doi: 10.1152/ajpregu.1993.264.1.R116. [DOI] [PubMed] [Google Scholar]
- Geary N. Pancreatic glucagon signals postprandial satiety. Neurosci Biobehav Rev. 1990 Fall;14(3):323–338. doi: 10.1016/s0149-7634(05)80042-9. [DOI] [PubMed] [Google Scholar]
- Gorczyca W., Gong J., Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res. 1993 Apr 15;53(8):1945–1951. [PubMed] [Google Scholar]
- Holst J. J. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33-69) of glicentin. Biochem J. 1982 Dec 1;207(3):381–388. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson J., Carlsson L., Edlund T., Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994 Oct 13;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., Clare-Salzler M., Tian J., Forsthuber T., Ting G. S., Robinson P., Atkinson M. A., Sercarz E. E., Tobin A. J., Lehmann P. V. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993 Nov 4;366(6450):69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K., Suzuki S., Ohashi S., Mukai H., Ohmori H., Murayama Y., Yamashita K. Comparison of the effects of glucagon-like peptide-1-(1-37) and -(7-37) and glucagon on islet hormone release from isolated perfused canine and rat pancreases. Endocrinology. 1989 Apr;124(4):1768–1773. doi: 10.1210/endo-124-4-1768. [DOI] [PubMed] [Google Scholar]
- Komatsu R., Matsuyama T., Namba M., Watanabe N., Itoh H., Kono N., Tarui S. Glucagonostatic and insulinotropic action of glucagonlike peptide I-(7-36)-amide. Diabetes. 1989 Jul;38(7):902–905. doi: 10.2337/diab.38.7.902. [DOI] [PubMed] [Google Scholar]
- Koranyi L., James D. E., Kraegen E. W., Permutt M. A. Feedback inhibition of insulin gene expression by insulin. J Clin Invest. 1992 Feb;89(2):432–436. doi: 10.1172/JCI115602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska Y. T., Villa-Komaroff L., Halban P. A. Islet B-cell dysfunction and the time course of recovery following chronic overinsulinisation of normal rats. Diabetologia. 1988 Aug;31(8):621–626. doi: 10.1007/BF00264771. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S., VOLK B. W. The effect of protracted glucagon administration on blood glucose and on pancreatic morphology. Endocrinology. 1958 Sep;63(3):359–371. doi: 10.1210/endo-63-3-359. [DOI] [PubMed] [Google Scholar]
- LOGOTHETOPOULOS J., SALTER J. M. Morphology and cytochemistry of alpha cells of the rabbit pancreas: effects of glucagon, insulin and infusions of glucose. Diabetes. 1960 Jan-Feb;9:31–37. doi: 10.2337/diab.9.1.31. [DOI] [PubMed] [Google Scholar]
- LOGOTHETOPOULOS J., SHARMA B. B., SALTER J. M., BEST C. H. Glucagon and metaglucagon diabetes in rabbits. Diabetes. 1960 Jul-Aug;9:278–285. doi: 10.2337/diab.9.4.278. [DOI] [PubMed] [Google Scholar]
- Le Sauter J., Geary N. Hepatic portal glucagon infusion decreases spontaneous meal size in rats. Am J Physiol. 1991 Jul;261(1 Pt 2):R154–R161. doi: 10.1152/ajpregu.1991.261.1.R154. [DOI] [PubMed] [Google Scholar]
- Le Sauter J., Noh U., Geary N. Hepatic portal infusion of glucagon antibodies increases spontaneous meal size in rats. Am J Physiol. 1991 Jul;261(1 Pt 2):R162–R165. doi: 10.1152/ajpregu.1991.261.1.R162. [DOI] [PubMed] [Google Scholar]
- Leonard J., Peers B., Johnson T., Ferreri K., Lee S., Montminy M. R. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol. 1993 Oct;7(10):1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Madsen O. D., Andersen L. C., Michelsen B., Owerbach D., Larsson L. I., Lernmark A., Steiner D. F. Tissue-specific expression of transfected human insulin genes in pluripotent clonal rat insulinoma lines induced during passage in vivo. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6652–6656. doi: 10.1073/pnas.85.18.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen O. D., Karlsen C., Blume N., Jensen H. I., Larsson L. I., Holst J. J. Transplantable glucagonomas derived from pluripotent rat islet tumor tissue cause severe anorexia and adipsia. Scand J Clin Lab Invest Suppl. 1995;220:27–35. [PubMed] [Google Scholar]
- Madsen O. D., Karlsen C., Nielsen E., Lund K., Kofod H., Welinder B., Rehfeld J. F., Larsson L. I., Steiner D. F., Holst J. J. The dissociation of tumor-induced weight loss from hypoglycemia in a transplantable pluripotent rat islet tumor results in the segregation of stable alpha- and beta-cell tumor phenotypes. Endocrinology. 1993 Nov;133(5):2022–2030. doi: 10.1210/endo.133.5.8404649. [DOI] [PubMed] [Google Scholar]
- Madsen O. D., Larsson L. I., Rehfeld J. F., Schwartz T. W., Lernmark A., Labrecque A. D., Steiner D. F. Cloned cell lines from a transplantable islet cell tumor are heterogeneous and express cholecystokinin in addition to islet hormones. J Cell Biol. 1986 Nov;103(5):2025–2034. doi: 10.1083/jcb.103.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura C., Chen L., Appel M., Alam T., Inman L., Hughes S. D., Milburn J. L., Unger R. H., Newgard C. B. Expression of reg/PSP, a pancreatic exocrine gene: relationship to changes in islet beta-cell mass. Mol Endocrinol. 1991 Feb;5(2):226–234. doi: 10.1210/mend-5-2-226. [DOI] [PubMed] [Google Scholar]
- O'Hare M. M., Shaw C., Swanston-Flatt S. K., Marcelli M., Buchanan K. D., Flatt P. R. Influence of a transplantable insulinoma on the pancreatic status of insulin and pancreatic polypeptide in the rat. Diabetologia. 1985 Mar;28(3):157–160. doi: 10.1007/BF00273864. [DOI] [PubMed] [Google Scholar]
- Ohlsson H., Karlsson K., Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993 Nov;12(11):4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Nielsen O. V. Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988 Oct;123(4):2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- Orskov C., Jeppesen J., Madsbad S., Holst J. J. Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991 Feb;87(2):415–423. doi: 10.1172/JCI115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSSON B., HELLMAN B. EFFECTS OF LONG TERM ADMINISTRATION OF GLUCAGON ON THE PANCREATIC ISLET TISSUE OF RATS AND GUINEA-PIGS. Acta Endocrinol (Copenh) 1963 Sep;44:139–149. doi: 10.1530/acta.0.0440139. [DOI] [PubMed] [Google Scholar]
- PETERSSON B., HELLMAN B. Long-term effects of restricted caloric intake on pancreatic islet tissue in obese-hyperglycemic mice. Metabolism. 1962 Mar;11:342–348. [PubMed] [Google Scholar]
- Peers B., Leonard J., Sharma S., Teitelman G., Montminy M. R. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994 Dec;8(12):1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- Petersen H. V., Serup P., Leonard J., Michelsen B. K., Madsen O. D. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10465–10469. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A., Suarez-Pinzon W. L., Shi Y., Morgan A. R., Bleackley R. C. DNA fragmentation is an early event in cytokine-induced islet beta-cell destruction. Diabetologia. 1994 Aug;37(8):733–738. doi: 10.1007/BF00404328. [DOI] [PubMed] [Google Scholar]
- Strubbe J. H., Wolsink J. G., Schutte A. M., Balkan B., Prins A. J. Hepatic-portal and cardiac infusion of CCK-8 and glucagon induce different effects on feeding. Physiol Behav. 1989 Oct;46(4):643–646. doi: 10.1016/0031-9384(89)90345-4. [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Yamato E., Noma Y., Tahara Y., Ikegami H., Yamamoto Y., Cha T., Yoneda H., Ogihara T., Ohboshi C., Hirota M. Suppression of synthesis and release of glucagon by glucagon-like peptide-1 (7-36 amide) without affect on mRNA level in isolated rat islets. Biochem Biophys Res Commun. 1990 Mar 16;167(2):431–437. doi: 10.1016/0006-291x(90)92041-w. [DOI] [PubMed] [Google Scholar]
- van Prooijen-Knegt A. C., Raap A. K., van der Burg M. J., Vrolijk J., van der Ploeg M. Spreading and staining of human metaphase chromosomes on aminoalkylsilane-treated glass slides. Histochem J. 1982 Mar;14(2):333–344. doi: 10.1007/BF01041225. [DOI] [PubMed] [Google Scholar]